Citation: Boyer K, Lund T, DcDough B, “The Trusted Tablet: a New Level of Supply Chain Security From Mass Digitalisation of Medicines”. ONdrugDelivery Magazine, Issue 103 (Dec 2019), pp 18-21.
Kelly Boyer, Trent Lund and Barry McDough describe a means of mass digitalising medicines to address the problem of illegal and unauthorised production of pharmaceutical products in a meaningful way.
THE CHALLENGE OF ILLEGITIMATE MEDICINES
Unauthorised and illegitimate production of medicines continues to jeopardise patient safety across the world. While regulators and manufacturers have made progress to secure global pharmaceutical supply chains, many of the predominantly packaging-based security approaches are ineffective.
The operational challenges of the modern pharmaceutical supply chain are well established. Globalisation, outsourcing and online retailing have significantly complicated the operating environment. As supply chains become more complex, a loss of control and visibility has occurred. Each node in the supply chain represents a potential point of failure where there is an opportunity for illegitimate products to enter. The incidence of counterfeit medicines, unauthorised generics, expired lots and diverted products have all become significant challenges.
“Serialisation and authentication of packaging on its own cannot be considered a dependable means of product verification. Relying exclusively on this approach provides healthcare professionals and patients with a false sense of security that the product within is genuine.”
The risk posed to public health and safety of illegitimate medicines is extremely worrying – particularly in the case of counterfeit products, which are often manufactured in squalid conditions. At best, they will be a placebo with no active pharmaceutical ingredients and can lead to therapeutic failure. At worst, they might contain heavy metals and even poisons, causing sickness and even death.
The WHO estimates that counterfeit medicines account for 10-30% of the market in developing economies and up to 1% in some developed economies.
In addition to the threat to patient safety, the cumulative commercial costs of unauthorised products is staggering. While the insidious nature of counterfeiting makes it difficult to determine the size of the problem, the pharmaceutical industry consensus is that 2% or more of top-line sales are impacted. Beyond this direct commercial impact, illegitimate medicines have a wider impact on the economy – damaging patient trust, brand reputation, tax revenue, investment and even innovation.
INDUSTRY RESPONSE TO ILLEGITIMATE MEDICINES
In response to external supply-chain threats, the pharmaceutical industry has sought to protect products through serialisation and other secure packaging solutions. While every effort helps, there are a number of shortfalls in relying on serialisation and other packaging features as a means of product authentication:
- Serialisation is not secure. Serialisation in itself is not a secure solution. Serialised data carriers can be reproduced with ease and en mass, especially where serialisation is sequential.
- Re-use of original packaging. Original packaging is retrieved, reused and resold having been filled with counterfeit, expired or unauthorised generic products.
- Packaging is being copied. The availability of high-tech, low-cost scanning and production technologies enable sophisticated replication of packaging by counterfeiters that is often indistinguishable from authentic packages.
- Repackaging. Pharmaceutical products are often removed from their original packaging and repackaged, rendering the original packaging security features obsolete.
For these reasons, serialisation and authentication of packaging on its own cannot be considered a dependable means of product verification. Relying exclusively on this approach provides healthcare professionals and patients with a false sense of security that the product within is genuine. In addition, the information systems that store serialisation data and information on provenance are typically not accessible by the general public.
A STEP TOWARDS THE DIGITALISATION OF MEDICINES
With the limitations of serialisation and packaging authentication in mind, a coalition of supply-chain partners – Colorcon, TruTags and PwC – came together to offer a new digitalisation approach to solving the challenges of illegitimate medicines. Whilst the benefits of digitalisation of medicines have been well documented, progress towards this goal has been inhibited by the economic and regulatory barriers created by the available technology solutions. The mass application of sensor technology directly on medicine doses is cost prohibitive for the majority of therapies, while the need for regulatory approval means that implementation can take several years.
This case study presents the findings of a supply-chain simulation in which innovative technologies are utilised to overcome the hurdles for on-dose security solutions mentioned above and illustrate a clear path towards the mass digitisation of medicines.
This simulation demonstrates how supply-chain actors and even patients can be empowered in the verification process, and how medicines can become trusted once more.
Three major technology elements were utilised in the simulation:
1. Application of Edible Barcodes
The first component of the system is on-product identifiers called TruTags®. TruTags are microtags — effectively, edible barcodes — that can be easily and economically incorporated onto tablets and capsules via existing coatings or inks (Figure 1).

Figure 1: TruTags are microtags — effectively, edible barcodes — that can be easily and economically incorporated onto tablets.
TruTags are made from microparticles of silicon dioxide, a material designated as Generally Recognised as Safe (GRAS) for ingestion and already commonly found in medicines. Because this material is readily present within tablets and capsules, the adoption of TruTags is relatively straightforward even for products that have already been launched on the markets.
During their manufacture, TruTags are encoded spectrally so they reflect light at a known spectral index. This spectral code can be directly associated with a product, a designated market, a production facility or even a batch.
In this supply-chain simulation, TruTags were mixed with a formulated Opadry® complete film coating system, from Colorcon, to create an intelligent coating. This coating was then simply applied to a batch of tablets using a standard coating process.
2. Authentication of Edible Barcodes
The second component of the system is the authentication technology. The authentication technology provides a link between the physical tablet and the digital backend. TruTags can be authenticated using either proprietary imagers or by using an application for commonly used cell phones (Figure 2).
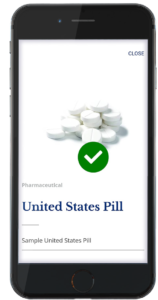
Figure 2: TruTags can be authenticated using either proprietary imagers or by using an application for commonly
used cell phones.
The authentication option used depends on the problem the brand is facing and who the company would like to be able to authenticate their products. Various stakeholders can be given the ability to authenticate, from quality assurance and security teams to law enforcement, to healthcare professionals and patients.
In addition to facilitating immediate authentication of medicines, anytime and anywhere, the application of cell phone technology affords an incredible opportunity for patient communication.
Pharmaceutical brands can communicate directly with their patient populations and personalise the treatment experience – monitoring patient compliance and treatment efficacy, all the while assuring the quality and safety of the medicines being consumed (Figure 3).

Figure 3: Pharmaceutical brands can monitor patient compliance and treatment efficacy.
3. The Digital Backend
The third component of the solution is a fully integrated, distributed ledger operated by PwC. The platform uses a Google technology called Trillian, a verifiable data structure that provides a transparent, immutable, and cryptographically verifiable transaction log (Figure 4). The platform integrates directly with the authentication application facilitating a secure link between the physical and digital world and delivering absolute supply-chain security and transparency.
As tablets are scanned with the application, the platform is instantly updated providing details on time, location and authentication result.
By setting up exception-reporting, brands can be automatically informed of instances of counterfeit, expired or diverted product.
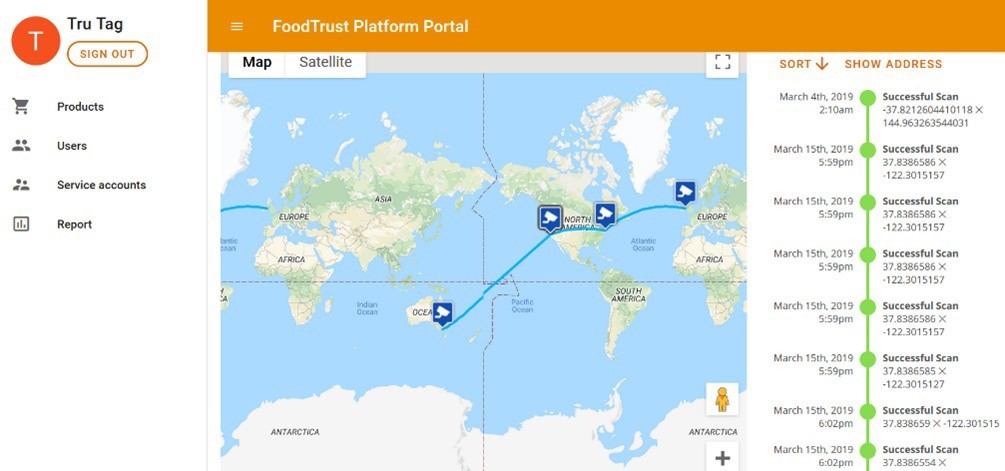
Figure 4: Google Trillian integrates directly with the authentication application facilitating a secure link between the physical and digital world.
THE SIMULATION
The supply-chain simulation of this technology was conducted over a six-month period between March and August 2019. A batch of 1,000 tablets were coated in a cGMP facility in Kapolei, HI, US, using three variants of an intelligent Opadry coating system, with TruTags included, to represent two different sales regions. Non-tagged coated tablets were also included amongst the tagged tablets to represent counterfeits.
The tablets were then sent to six known parties in the US, Australia and Europe who simulated regional wholesalers and patients. Each party was provided access to the cell phone application and requested to authenticate the tablets distributed to them.
Over 5,000 authentication events were recorded on the PwC platform over this period. Information on user, scan time, location and authentication outcome were all recorded.
Untagged tablets were successfully identified, as were tablets being authenticated outside of their designated sales markets. No false positives were recorded during the trial.
CONCLUSION
In this case study, the coalition of partners successfully demonstrated how individual tablets can be digitalised, linked to a distributed ledger platform and authenticated by supply-chain patients, providing absolute supply- chain security and transparency. By enabling instant authentication, anytime, anywhere, the pharmaceutical industry can secure the supply chain and, in doing so, safeguard their patients and their brands.
Beyond improved supply-chain integrity, this innovation has the potential to connect brands directly with their patient populations. This can help drive engagement between healthcare providers and patients, with the potential to improve patient outcomes.
TRUTAG and TRUTAGS are registered trademarks of TruTag Technologies, Inc. OPADRY and COLORCON are registered trademarks of BPSI Holdings, LLC. PwC refers to the PwC network and/or one or more of its member firms, each of which is a separate legal entity.


