Citation: Donnelly A, Uddin S, “Addressing the Challenges of Viscous Injectable Administration”, ONdrugDelivery, Issue 120 (May 2021), pp 54–57.
Andrew Donnelly and Shahid Uddin look at the challenges of self-administration of injectable viscous formulations and the technology that is helping to overcome these issues.
“The intrinsic nature of many high-viscosity formulations means that only so much can be done to optimise them for use in standard autoinjectors. This is driving the need for alternative devices that can offer a viable alternative when reducing viscosity is not an option.”
To date, one of the key issues holding back the widespread adoption of self-administration for injectable formulations is that many of the available devices are not able to deliver the drug through a needle fine enough to be acceptable to the patient, particularly for viscous formulations. A wide-diameter needle is perceived as uncomfortable or even painful, to use, making many patients reluctant to use them. This reluctance can all too easily translate into non-compliance, with repercussions for the medicine’s efficacy and, ultimately, the patient’s health.
With this in mind, it is clear that there is a pressing need to create more user-friendly devices, even when the formulation is viscous. To achieve this, significant challenges must be overcome during drug development to improve ease of administration, and steps must be taken to create devices that can cater for more challenging formulations.
WHAT MAKES A LIQUID VISCOUS?
A liquid with high internal resistance to flow is described as having high viscosity. This internal resistance is created when the molecules move past one another, for example when the liquid is poured out of a container. Liquids made up of small, relatively simple molecules tend to have low viscosity, whereas liquids with larger, more complex long-chain molecules have a much higher viscosity. This is because the molecular chains get tangled up in each other as the liquid moves.
Viscosity is also governed by the strength of a liquid’s intermolecular forces, especially the shapes of its molecules. Liquids with molecules that can form bonds with each other are usually more viscous. Honey, which mostly comprises of relatively small glucose and fructose molecules, is a good example of a liquid that owes its viscosity to internal bonding.
Liquid drug formulations can have different viscosities, depending on the nature of their APIs, the solvents used or the release profile they have been designed to achieve. The more viscous drug formulations in common use include:
- Formulations containing high concentrations of large molecules. Biologics, including monoclonal antibodies (mAbs), are a key example of formulations containing large, long-chain molecules. The nature of these therapies means that they often need to be delivered by injection. The impracticalities of intravenous administration mean that subcutaneous routes are more desirable when it comes to developing biologic treatments for self-administration. However, the volume of formulation that can be delivered in a single dose with a syringe or autoinjector is limited to 2 mL or less. The need for higher doses is driving the need for higher concentrations of the drug, especially when it comes to mAbs. This increase in concentration generally leads to formulations with much higher viscosities in the range of 20–100 cP.
- Formulations designed to provide sustained or controlled release. For many therapeutics that are rapidly cleared from the body, it is desirable to control the rate of release of the active agent from the site of injection, thus reducing the need for multiple doses. However, many of these controlled-release formulations include polymers with a high molecular weight, which render the formulations extremely viscous. The viscosity of these formulations can be more than 1000 cP.
- Non-aqueous formulations. For some formulations, the solvent itself is highly viscous. Oil-based formulations, for example, may be needed to generate controlled-release characteristics or as solvents for drugs that are poorly water soluble. The viscosity of these formulations is similar to the viscosity of the oil – for example, the viscosity of castor oil is in excess of 2000 cP.
That said, it is clear that there are limitations on how much drug formulations, particularly those for biologic treatments, can be improved to reduce their viscosity. The intrinsic nature of many high-viscosity formulations means that only so much can be done to optimise them for use in standard autoinjectors. This is driving the need for alternative devices that can offer a viable alternative when reducing viscosity is not an option.
“Despite the development and manufacturing challenges posed by the viscosity of biologic drug formulations, they remain an exciting area for drug innovation. Steps can be taken not only to overcome manufacturing efficiency issues, but also useability issues for patients.”
THE COMPLEXITIES OF MANUFACTURING HIGHLY VISCOUS FORMULATIONS
Increasing the concentration, and thus the viscosity, of biologic formulations has ramifications for the manufacturing process and the product’s storage requirements, as well as how it is ultimately administered to patients. One example is the biologic therapies used to treat immunological and genetically related diseases. As previously mentioned, these are often delivered subcutaneously, which means the formulations must contain high concentrations of drug substance to overcome the limited volume of drug that can be administered in a single dose. To achieve these high concentrations within a formulation, diafiltration is used – a process that separates out different-sized protein molecules within a preformulation via micro-molecule permeable filters in order to concentrate quantities of the desired protein.
However, diafiltration can pose challenges when it comes to manufacturing a formulation, particularly with regard to stability and aggregation, as protein-protein interactions become more likely when concentration increases. Ordinarily, instability and aggregation occur because diafiltration is a dynamic operation in which protein concentration increases by volume reduction and buffer matrix changes.1 This instability can have significant repercussions in terms of product quality and shelf life. As such, the diafiltration process requires extensive expertise and should be highly controlled to ensure product quality is not impacted (Figure 1).
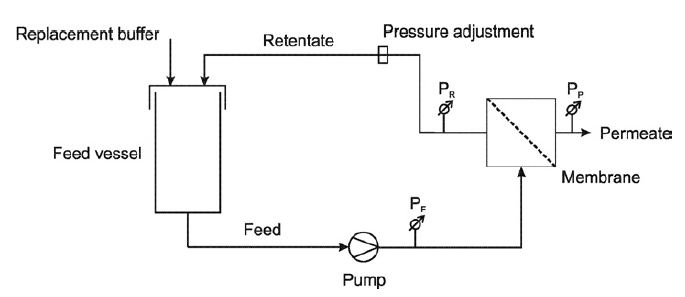
Figure 1: Representation of diafiltration setting.2 (Source: WikipediaDorian).
“By taking the patient experience into account from the beginning of the parenteral drug development stage, we can ensure that we do our part to create easier-to-use injectable drugs.”
Manufacturing scalability is also a key concern with diafiltration. It is not a simple case of expanding the filter membrane surface area as the proportion of protein being processed is increased. The high viscosity of high protein concentrations increases operating pressure in diafiltration.3 This can reduce the protein-loading capacity of the filter, which means that the protein load ratio must be decreased to achieve the same performance at higher scale.
In addition to these scalability issues, viscous biologics can lead to costly material waste during the filtration process, leaving behind unused but viable protein in the filters. Although this problem may be prevented by oversizing the membrane, a setup that uses low-concentration operations necessitates changes that promote high-concentration operations. Using a membrane with a low molecular weight cut-off may reduce protein loss during filtration.4
Furthermore, the high mechanical stress experienced during diafiltration can cause irreversible protein aggregation. This is a phenomenon that sees protein molecules group together into insoluble masses, negatively impacting their effectiveness in the finished formulation, as well as potentially increasing viscosity further.
To enable successful development of high-concentration biologics, a number of excipients can be added to formulations to stabilise proteins by suppressing aggregation and surface adsorption. In liquid formulations, buffers with phosphate or histidine and a small amount of detergent can be useful. Excipients for lyophilised formulations include sucrose, trehalose, mannitol and glycine, among others. However, these add complexity and cost to the formulation development process, further impacting on manufacturing efficiency (Figure 2).
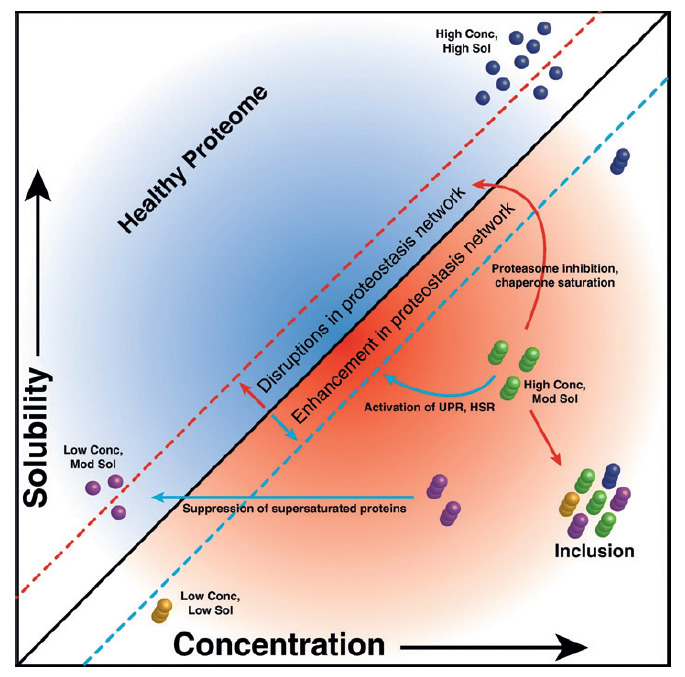
Figure 2: Protein aggregates.5 (Source: Ciryam P, Antalek M, Cid F, et al, “A metastable subproteome underlies inclusion formation in muscle proteinopathies”. Acta Neuropathol Commun, 2019, Vol 7(197).)
On top of challenges when producing stable, high-concentration biologic formulations, viscosity can cause complications during processing. The transfer of viscous solutions via pumping can be challenging and can generate high back pressures during the filtration process. The physical stresses that these processes apply to the solution can also create stability issues. Clogging of the filling needle can occur as solute is deposited in the needle with high drying rates, if temperature and humidity are not controlled.6 Furthermore, the process-yield issues that this creates lead to significant economic losses.
These issues can be daunting for drug companies, creating a false perception that the overall profitability of parenteral drug products using viscous drug formulations may be lower than desirable. It may also lead them to believe wrongly that their options are limited when it comes to designing more patient-friendly parenteral drug products for self-administration, impacting on the adoption of autoinjectors.
ACHIEVING DRUG DELIVERY SUCCESS
Despite the development and manufacturing challenges posed by the viscosity of biologic drug formulations, they remain an exciting area for drug innovation. Steps can be taken not only to overcome manufacturing efficiency issues, but also use ability issues for patients. Enhancing the effectiveness of the delivery device used for viscous medications can go a long way towards creating a better experience for patients, with or without changes to the formulation.
Viscous formulations need to be delivered with relative ease and cause limited pain, which is a challenge using standard devices. Many traditional syringes are only able to deliver viscous formulations by using a needle with a wider diameter, as this can significantly reduce the pressure required to administer the drug. These wider needles can cause unpleasantness for patients, particularly if they are self-administering, since they are not trained to manage the potential discomfort in the same way as healthcare professionals. This can lead to a poor patient experience, with potential negative consequences for patient adherence.
However, advances in technology can overcome this issue, to the benefit of the patients self-administering the drug. One such innovation involves devices that use a liquefied gas, rather than a spring, to push the formulation through the needle. This can allow greater pressure to be exerted to push the drug through a fine needle, while minimising the risk of the needle breaking, the device stalling or causing undue discomfort for the patient. As a result, they can eliminate the need for unpleasant wide-diameter needles.
One final hurdle that needs to be overcome is the design of the primary formulation container. Many standard autoinjector devices utilise glass prefilled syringes, which can be susceptible to cracking when high pressures are applied to deliver a viscous drug formulation. Improvements to container design in recent years have significantly reduced the likelihood of this happening, but there is still a risk.
An option to overcome this issue is to use polymeric primary containers, such as those based on cyclic olefin polymer and copolymer (COP/COC). These can better withstand the pressures experienced during the administration of viscous drugs. However, there are concerns with stability and leachables for some products, which means they are not yet a universal solution to the problem of drug container breakage.
TIME TO ACT: CREATING A BETTER USER EXPERIENCE
The growth of viscous formulations on the market shows no sign of slowing down, with many new treatments in development. Consequently, the need to keep patient-centricity front of mind grows ever more pressing, especially if we are to continue to develop drugs designed for self-administration. By taking the patient experience into account from the beginning of the parenteral drug development stage, we can ensure that we do our part to create easier-to-use injectable drugs. There may be limitations in terms of how far we can improve the formulations of drugs to reduce their viscosity, but there are many opportunities to enhance the design of the devices that deliver the finished formulations.
There are third-party device experts available who can support drug companies in developing more useable, patient friendly injectable treatments. By working with outsourced partners that specialise in the creation of high-quality autoinjector devices, drug companies can ensure that they have the help and guidance they need to overcome the administration and manufacturing challenges of viscous injectable drug formulations.
By working with such partners, companies can ensure that even viscous drugs are easy for patients to administer themselves with minimal discomfort, allowing them to truly harness the benefits of self-administration in the parenteral space. These will offer greater patient convenience and the liberation of healthcare professionals from the time-consuming need to administer injections on their patients’ behalf. As such, better delivery devices have the potential to optimise patient adherence, transforming their health, while enhancing efficiency for resource-constrained public healthcare systems.
REFERENCES
- Arunkumar A et al, “Effect of channel-induced shear on biologics during ultrafiltration/ diafiltration (UF/DF)”. J Membr Sci, 2016, Vol 514, pp 671–683.
- Kovács Z, Discacciati M, “Modelling of Multi-Step Microfiltration Process for Solvent Exchange”. Hung J Ind Chem, 2008, Vol 36(1–2), pp 65–69.
- Holstein M et al, “Strategies for high‐concentration drug substance manufacturing to facilitate subcutaneous administration: A review”. Biotechnol Bioeng, 2020, Vol 117(11), pp 3591–3606.
- Sahin E, Deshmukh S, “Challenges and Considerations in Development and Manufacturing of High Concentration Biologics Drug Products”. J Pharm Innov, 2019, Vol 15(1), pp 1–13.
- Ciryam P, et al, “A metastable subproteome underlies inclusion formation in muscle proteinopathies”. Acta Neuropathol Commun, 2019, Vol 7(197), pp 1–14.
- Haslip S, “Challenges in filling High Concentration, High Viscosity Drug Product Formulations”. PDA, 2017 Universe of Pre-filled Syringes and Injection Devices 2017.

