Citation: Kraus R, Woesthuis W, “Advanced Drug Delivery for Dry Eye Treatment”. ONdrugDelivery Magazine, Issue 104 (Jan 2020), pp 4-6.
Rouven Kraus and Wilfried Woesthuis discuss Aero Pump and Medspray’s new open-eye spray for the treatment of dry eyes.
If you go to a drugstore and look for a medicine to treat your dry eyes, you will see that such moisturising products are usually packed in eye droppers. But the use of eye drops can be challenging, especially for elderly people. The patient has to tilt their head back, hold their eye open and squeeze the bottle so that the drop goes into the eye.
“The new eye spray can be applied from a close distance and from the front, directly onto the open eye, without causing a blink reflex.”
Eye sprays can be used as an alternative for patients who feel unable to use eye drops. The vast majority of eye sprays currently available on the market are applied to the closed eye. These rehydrating eyelid sprays are often based on a liposomal formulation, soy lecithin or sodium hyaluronate, offering hydrophilic properties.
Dry eye disease is divided into aqueous-deficient dry eye (20%) and evaporative dry eye (80%).1 Liposomal eye sprays are specifically designed to address evaporative dry eye symptoms. Liposomes stabilise the lipid layer at the lacrimal film to improve the hydration of the ocular surface. The spray is applied onto the closed eyelid. The eye is kept closed for a few seconds, allowing the liposomes to reach the lid edge. Opening the eye spreads the artificial tear film across the entire eye surface.
But why not apply the formulation from a close distance onto the naked eye, by spraying an extremely fine and soft mist that avoids the blink reflex of the eye?
A CONVENIENT WAY TO TREAT DRY EYE
Aero Pump now offers such an alternative that is completely new to the market. Besides its preservative-free multi-dose eye dropper using 3K technology, Aero Pump has developed, together with its partner Medspray, innovative drug delivery technology for a topical ocular treatment.
The new eye spray can be applied from a close distance and from the front, directly onto the open eye, without causing a blink reflex. The unique ultra-soft fine mist delivers such small droplets the patient barely feels it (Figure 1).

Figure 1: The eye spray targeting the open eye without blink reflex.
The new open-eye spray is a preservative-free multi-dose device offering metered dose applications directly to the surface of the eye. The spray delivers an accurate 45 μL dose (dosage adaption possible).
Comfort and ease of use played a crucial role in the product development. The open eye spray completes Aero Pump’s range of ophthalmic drug delivery devices, offering an alternative to eye droppers.
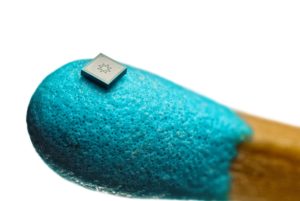
Figure 2: The tiny spray nozzle unit (SNU) in comparison with a match head for scale.
The innovation is based on a horizontal pump in combination with a micro- and nanotechnology-based spraynozzle that creates an ultra-soft, slow-moving fine mist by microdiffusion. The horizontal pump is designed to release the aqueous formulation first after a defined pressure. Then the liquid is accelerated and pushed through a tiny spray nozzle unit (SNU), developed by Medspray. The SNU (shown in Figure 2 positioned on the head of a match for scale) is composed of multiple micro-holes. In comparison, existing eye sprays only have an insert with just one output.
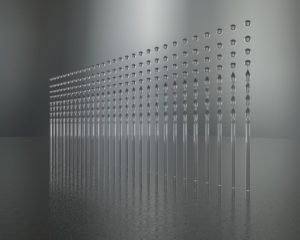
Figure 3: Medspray’s spray nozzle unit operates via the Rayleigh principle, whereby a liquid jet spontaneously breaks up into droplets.
Based on the Rayleigh principle, whereby a liquid jet spontaneously breaks up into droplets, Medspray’s patented SNU breaks up jets into mono-disperse droplet trains (Figure 3). As an example: a 5 μm hole creates a jet, breaking up into 10 μm droplet trains. The diameter of the droplets is twice the size of the orifice.
Medspray’s SNU was developed at the nanolab of the University of Twente (Enschede, The Netherlands). The manufacturing process is based on established micro- and nanotechnology. The orifices of the spray nozzles are extremely tiny (1–30 μm). For reference, a human hair is about 70 μm thick. Engineering the size (1–30 μm), number of holes (1–200), vectors and geometric pattern means the spray characteristics can be tailored to different applications to meet specific industry partner requirements.
COMPATIBILITY WITH A WIDE RANGE OF OPHTHALMIC SOLUTIONS
Medspray conducted extensive formulation acceptance tests with several ophthalmic products from the market. The results of such studies show that the new eye spray is compatible with many well-known eye drop brands. The formulation has to be in an aqueous viscosity level. Even some formulations in the higher viscosity level can be applied through the SNU. The adjustable pore size of the SNU allows it to cover a wide range of ophthalmic solutions.
The formulation acceptance tests (FAT) were performed with several well-known ophthalmic brands for dry eye relief. Low-viscous as well as high-viscous products in the range of up to 125 mPas (e.g. hyaluronic acid) passed the FAT with Medspray’s SNU. So it suggests the new eye spray is compatible with a wide range of ophthalmic solutions.
MAXIMUM PATIENT COMFORT
With the ultra-soft, slow-moving fine mist generated by the SNU – that even sensitive users will barely feel – the new eye spray can be easily administered onto the open eye. Furthermore, it has a great advantage over eye drops – it can be applied in a comfortable head position without the need to tilt the head back. This is especially beneficial for infants and the elderly.
The droplets are so small that it avoids smudging of make-up, even from a close distance. The new eye spray can also be used with contact lenses.
The device is protected from microbiological contamination, so there is no need to add preservatives to the formulation. The pump technology offers propellant-free spray solutions so it is also eco-friendly. Aero Pump is setting up serial production of the eye spray this year at its facility in Hochheim (Germany).
REFERENCES
- Dausch D et al, “Comparative Study of Treatment of the Dry Eye Syndrome due to Disturbances of the Tear Film Lipid Layer with Lipid-Containing Tear Substitutes”. Klinische Monatsblätter für Augenheilkunde, 2006, Vol 223(12), pp 974–983.


