Citation: Trémeau S, Nicolas M, Leszczynski M, “BD Hylok™ – Glass Prefillable Syringe for IV Applications”. ONdrugDelivery, Issue 117 (Mar 2021), pp 59-64.
Sophie Trémeau, Maxime Nicolas and Myriam Leszczynski discuss BD Hylok™, a prefillable glass syringe for intravenous applications, including the rigorous testing BD Hylok™ has undergone and the regulatory support BD is able to provide to its pharmaceutical partners.
“The aim was to allow hospitals to take advantage of glass prefillable syringe formats for IV applications while mitigating the risks previously associated with glass syringes in relation to connector incompatibility. The BD Hylok™ glass prefillable syringes for IV precisely addresses the need for compatibility with commonly used NLADs.”
Several factors motivate pharmaceutical companies and hospitals to use glass prefilled syringes for intravenous (IV) applications. By switching from vials and ampules to a glass prefillable syringe, hospitals have the potential to reduce medical errors,1,2 improve workflow efficiency3 and positively impact return on investment for hospital stakeholders.4 Moreover, data from a study conducted by BD suggests that approximately 92% of hospital stakeholders are willing to use prefillable syringe for multiple drugs.5
For pharmaceutical companies, transitioning a fixed-dose drug from a vial or ampule format to a prefillable syringe can serve as a differentiation strategy in a market dominated by vials and ampules.6,7 The benefits of glass as a prefillable syringe material include the inertness of the material and, in some cases, steam sterilisation resistance in the fill/finish process.8 However, glass prefillable syringe formats for IV applications have historically experienced some challenges that needed to be addressed in order for pharmaceutical companies and hospitals to be able to access these benefits without reservation.
OVERCOMING THE LIMITS OF GLASS PREFILLABLE SYRINGE FOR IV APPLICATIONS
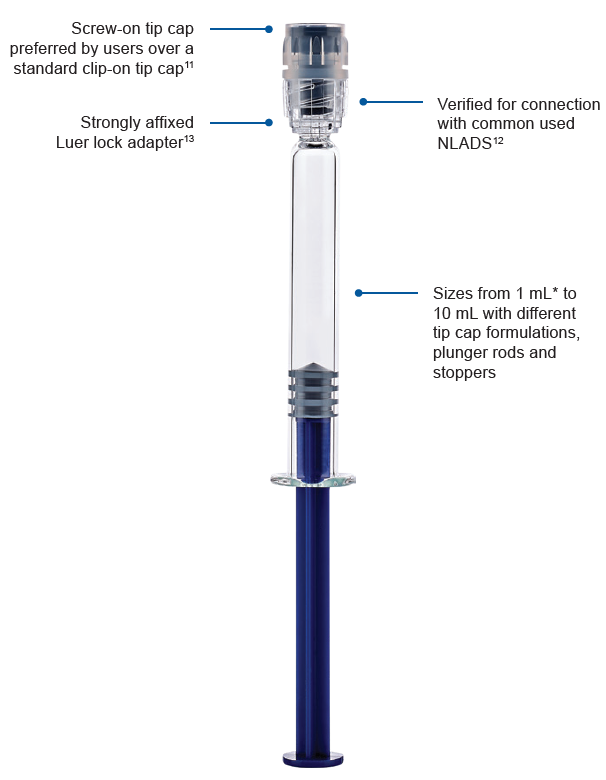
Figure 1: Connection performance features of BD Hylok™.
*1 mL and 3mL formats are available. Other sizes from
1.25 mL to 10 mL are currently under development.
Health authorities have indicated cases in which glass prefillable syringe have become clogged and malfunctioned when connecting to pin-activated IV needleless access devices (NLADs),9 including in emergency situations. Indeed, incompatibility between the syringe and the connector can result in the NLAD becoming clogged or the syringe tip being broken.9
To better understand these issues, BD conducted research in the US, UK, France and Germany.10 The results showed that 27% of the clinicians surveyed have experienced the prefillable syringes and intravenous line becoming disconnected, and 71% of clinicians who experienced these disconnections consequently experienced drug leakage.10
To address these issues, BD designed a glass prefillable syringe with connection performance specifically verified for IV applications.11 The aim was to allow hospitals to take advantage of glass prefillable syringe formats for IV applications while mitigating the risks previously associated with glass syringes in relation to connector incompatibility. The BD Hylok™ glass prefillable syringe for IV (Figure 1) precisely addresses the need for compatibility with commonly used NLADs (Figure 2).12
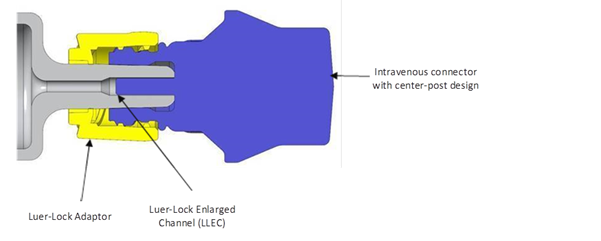
Figure 2: The LLEC of BD Hylok™ facilitates connection with commonly used NLADs.12
BD HYLOK™
The BD Hylok™ glass prefillable Luer syringe is designed for the administration of IV drugs. Indeed, the BD Hylok™ Luer lock adapter (LLA) resists an average pull-out force three times higher and a rotational torque five times higher than those of comparable products (Figures 3 and 4).13
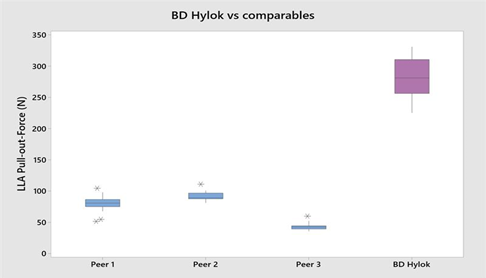
Figure 3: BD Hylok™ LLA resists a pull-out force three times higher than that of market comparables.13
BD Hylok™ design features include:
• A new LLA thread design
• LLA bonding technology
• A Luer lock enlarged channel (LLEC).
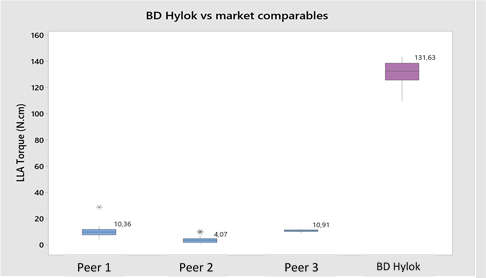
Figure 4: BD Hylok™ LLA resists rotational torque five times higher than that of market comparables.13
RIGOROUS TESTING
“Switching to a prefillable syringe can create clinical and economic value for healthcare providers, while differentiating the drug-device combination in a space dominated by vials and ampules.”
Verification of BD Hylok™ took place in accordance with ISO standards, including ISO 11040-4 and ISO 80369-7, covering the product connectivity. The tests cover all BD Hylok™ syringe sizes as the tip and LLA designs are the same.14,15 For design verification, the tests were performed on samples at 25°C, with and without ship tests (ISTA 3E), and filled with water for injection (after ethylene oxide and before steam sterilisation).
Verified for Steam Sterilisation
BD Hylok™ is suitable for both ethylene oxide (EtO) and steam sterilisation.16 Typically, EtO sterilisation is conducted by BD and steam sterilisation is conducted by the pharmaceutical company filling the syringe. In one study, pull-out force and rotational torque performance were tested on BD Hylok™ after two EtO and two steam sterilisation cycles at 121°C, for 20 minutes.13
In another study, market comparables were subjected to a typical process of one EtO and two steam sterilisation cycles at 121°C for 20 minutes.13 After EtO sterilisation, conditions were simulated in an environment where the glass syringe was prefilled and the LLA was subjected to pull-out force/rotational torque. After steam sterilisation, the resistance of the prefillable syringe to pull-out force and rotational torque was verified by subjecting the adapter to forces exerted in the act of screwing on the needle.
Compatibility with Commonly Used ISO Connectors
Compatibility with commonly used NLADs was verified through a series of tests evaluating the fluid path (absence of clogging) and connection tightness. BD Connecta™ and BD Microlance™ were tested relative to ISO 80369-7 whereas other connectors were tested relative to ISO594-2.12
Three tests referencing ISO80369-7 May 2013 draft 6.1.2. and ISO594-2: 1998 were used to verify the tightness of the connector to the BD Hylok™:17
• A pressure decay test verified the tightness of the connection between the syringe LLA/tip and the connector.17 An ISO connector or an NLAD was assembled on the syringe with a defined axial force and torque.
• A sub-atmospheric test verified the integrity of the connection between the syringe and the connector.17 An ISO connector or NLAD was fitted on the syringe with a defined axial force and torque. The connection was then exposed to sub-atmospheric pressure and evaluated for leakage.
• A stress cracking test verified that the connection between the syringe and the connector could withstand stress at room temperature.17 At the end of the time period, the connection was exposed to sub-atmospheric pressure and evaluated for leaks.
BD Hylok™ passed all three of the tests cited.17
Robust Connection Between Syringe and Connector
Three tests were used to verify the ability of BD Hylok™ to maintain the connection between the connector and the syringe tip, referencing ISO 80369-7 May 2013 draft 6.1.2. and ISO 594-2:1998:
• A connector unscrewing torque test verified that the connector did not disconnect from the syringe tip during injection.17
• The connector separation force test ensured that the connection with the Luer lock could withstand an axial load of 35 N.
• An overriding torque test confirmed the Luer lock’s capacity to resist override of the threads or lugs of the connector when subjected to over-torque.17
Luer Lock Robustness
“BD provides a robust and extensive data package to support development and registration of any combination products using BD Hylok™. The package includes, but is not limited to, quality statements, summaries of human factors user studies, product usage instructions, performance assessments and regulatory support.”
The robustness of the BD Hylok™ Luer lock was verified through tests evaluating the LLA’s resistance to pull-off forces, and its resistance to rotation.13 The tests referenced ISO 11040-4:2015 §6.5.3.5 and ISO 11040-4:2015 §6.5.3.6., respectively:
• An LLA pull-off force test verified that the LLA will not disconnect from the tip under an axial load of 22 N.
• An LLA dismantling torque test verified that the LLA will not disconnect from the tip under torque.13
REGULATORY FRAMEWORK FOR EUROPEAN MARKETS
BD Hylok™ prefillable syringes filled with drugs or biologics are considered an integral drug-device combination (DDC) in Europe. According to Article 117 of the European Medical Device Regulation (MDR) 2017/745, an integral DDC has to be assessed by an MDR-accredited notified body (NB), which should focus on the safety and performance of the device part of the DDC. The official date for implementation of European Medical Device Regulation, article 117, has been postponed by one year to May 26th, 2021, due to the covid-19 sanitary context.18
The applicable General Safety and Performance Requirements (GSPR) Annex I of the EU MDR are part of the design input specifications of BD Hylok™, which means that BD will be able to provide evidence of safety and performance quality to its partners to help them build their own GSPR-compliant packages for the DDC.
In cases where BD Hylok™ would be introduced as a post-approval change, an NB’s opinion should be submitted as part of the variation/extension application, as per section 2.6 of the Q&A document from the EMA (EMA/37991/2019 Rev.1 from October 21st, 2019).19
REGULATORY CONSIDERATIONS WHEN CONVERTING FROM VIAL TO PREFILLABLE SYRINGE
Converting from vials to prefillable syringes is considered to be a significant change that requires a variation and/or extension of the marketing authorisation in Europe and a prior approval supplement for the US market. From a regulatory perspective, the impact of the container closure system (CCS) change needs to be assessed and shown to not affect the quality, safety or efficacy of the DDC.20,21 The following items must be considered:
- Biocompatibility testing
- Impact on product quality due to container interaction or new manufacturing process
- Comprehensive stability testing
- Extractables and leachables
- Shipping studies
- Preclinical or clinical studies
- Human factors studies
- Labelling.
COMPREHENSIVE DEVELOPMENT AND REGISTRATION SUPPORT
BD provides a robust and extensive data package to support development and registration of any combination products using BD Hylok™. The package includes, but is not limited to, quality statements, summaries of human factors user studies, product usage instructions, performance assessments and regulatory support. This package provides comprehensive support to enable a smooth transition to BD Hylok™, either from an existing prefillable syringe or adoption in the case of a new product launch.
CONCLUSION
Switching to a prefillable syringe can create clinical1–3 and economic4 value for healthcare providers, while differentiating the DDC in a space dominated by vials and ampules. To meet this market need, the design and rigorous validation of BD Hylok™ enables hospitals to adopt glass prefillable syringes for their IV delivery needs with confidence.
REFERENCES
- Vogl TJ, Wessling J, Buerke B, “An observational study to evaluate the efficiency and safety of ioversol pre-filled syringes compared with ioversol bottles in contrast-enhanced examinations”. Acta Radiol, 2012, Vol 53(8), pp 914–920.
- Adapa RM et al, “Errors during the preparation of drug infusions: a randomized controlled trial”. Br J Anaesth, 2012, Vol 109(5), pp 729–734.
- Subhi Y, Kjer B, Munch IC, “Prefilled syringes for intravitreal injection reduce preparation time”. Dan Med J, 2016, Vol 63(4), A5214.
- Benhamou D et al, “Ready-to-use pre-filled syringes of atropine for anaesthesia care in French hospitals – a budget impact analysis”. Anaesth Crit Care Pain Med, 2017, Vol 36(2), pp 115–121.
- “Drivers of use of prefilled syringes”. Internal study, 2018, Becton, Dickinson and Company.
- “Considerations and Options for Prefilled Syringes”. White Paper, 2019, Baxter Healthcare Corporation.
- Data taken from IQVIA dataset, extracted Dec 2019.
- “Hylok value proposition confirmation”. Internal study, 2017, Becton, Dickinson and Company.
- “Connection problems involving certain needleless pre-filled glass syringes containing adenosine and amiodarone”. US FDA Safety Announcement, 2011.
- “Understanding needs of ICU Nurses, anaesthesiologists, risk/quality managers and infections control nurses in IV drug delivery in Acute care in relation to Prefilled Syringes (PFS) and the value associated with PFS”. Internal study, 2017, Becton, Dickinson and Company.
- “Simulated Human Factors Validation study of BD Hylok™ connectivity”. Internal Report, 2017, Becton, Dickinson and Company.
- “BD Hylok™ IV connectors and needles compatibility”. Internal Report, 2018, Becton, Dickinson and Company.
- “BD Hylok™ and BD Hylok™ competitor syringes performance evaluation”. Internal Report, 2018, Becton, Dickinson and Company.
- “ISO 80369-7:2016 Small-bore connectors for liquids and gases in healthcare applications – Part 7: Connectors for intravascular or hypodermic applications”. ISO, published 2016.
- “ISO 11040-4:2015 Prefilled syringes — Part 4: Glass barrels for injectables and sterilized subassembled syringes ready for filling”. ISO, published 2015.
- “BD Hylok™ Design verification results”. Internal Report, 2019, Becton, Dickinson and Company.
- “BD Hylok™ functional performances study”. Internal Report, 2018, Becton, Dickinson and Company.
- “Regulation (EU) 2020/561”. Official Journal of the European Union, published 2020.
- “Questions & Answers on Implementation of the Medical Devices and In Vitro Diagnostic Medical Devices Regulations ((EU) 2017/745 and (EU) 2017/746)”. European Medicines Agency Document, 2019.
- Soikes R, “Moving from Vial to Prefilled Syringe”. PharmTech, 2009, Vol 2009 Supplement, Issue 5. 21. Soikes R, “Shoot for Share! From Vial to Pre-filled Syringe”. Webinar, Jun 2009, Baxter BioPharma Solutions.


