Citation: Johnson R, “Formulation Options in Nasal Drug Delivery”. ONdrugDelivery, Issue 119 (Apr-May 2021), pp 8–13.
Richard Johnson discusses the advantages of nasal delivery and why there is growing interest in this route of administration, as well as considering some of the challenges that may arise during the development of nasal formulations for use in the clinic, and the regulatory and testing requirements associated with them.
This article was first published as a blog series on Upperton Pharma Solutions’ website.
The anatomy and physiology of the nasal cavity creates a number of unique opportunities for the successful delivery of drugs and vaccines. The target can be local, systemic or direct to the central nervous system (CNS). This utility has resulted in growing interest from scientists looking to administer both therapeutic agents and vaccines via the nasal route. This interest has been further fuelled by a number of factors, not least the surge in interest in delivering covid-19 prophylactic treatments and vaccines to the nasal cavity, the site of first infection.
Devices delivering drugs or vaccines into the nasal cavity can generally be divided into those delivering either a liquid or dry powder formulation. There has been a surge in interest in nasal delivery for both of these formulation approaches.
EXPLOITING NASAL PHYSIOLOGY FOR DRUG DELIVERY
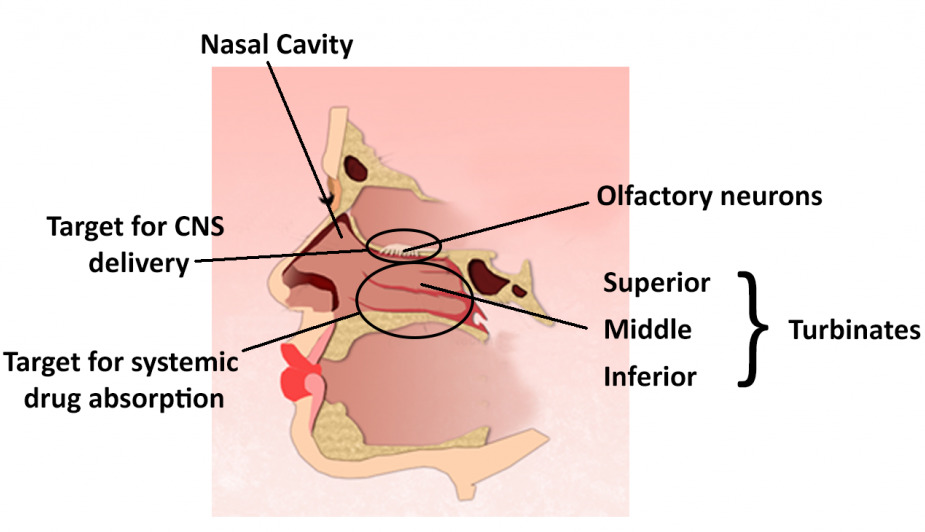
Figure 1: Regions of the nasal cavity.
In order for the nose to both smell and breathe, the human nasal cavity has developed a number of unique physiological features (Figure 1). These features can in turn be exploited for drug delivery purposes. The nostrils mark the entrance into the nasal cavities, which narrow to a point called the nasal ostium. The septum separates the two cavities, which extend, on average, 12–14 cm from the nostrils to the junction between the nose and pharynx. This junction is called the nasopharynx. The nasal-associated lymphoid tissue (NALT), an area that may be associated with inducing mucosal immunity, is located in the nasopharynx.
Within the nose itself, the main nasal passage is further divided by three projections from the septum called turbinates. These turbinates are designed to maximise contact between the air and mucosal surface. The inferior, middle, and superior turbinates are highly vascularised with relatively thin membranes. The result is an increase in the total surface area of the nasal cavity to 150 cm², making them an ideal target for systemic drug delivery. Other physiological targets for delivery are the olfactory and trigeminal nerves which innervate the nasal cavity. These neurones offer a potential target for nose-to-brain delivery and represent around 10% of the total nasal surface area.
WHAT ADVANTAGES DOES NASAL DELIVERY OFFER?
“One of the most obvious advantages of nasal delivery is the rapid absorption across the nasal membranes into the systemic circulation, resulting in fast onset of action. This means that systemically acting nasal products to be easily and rapidly administered for acute indications.”
Nasal delivery offers a number of advantages for certain classes of drug compounds and vaccines. These advantages include:
- Fast onset of action (rapid absorption)
- Potential for local, systemic and CNS delivery
- High levels of patient acceptance
- Improved bioavailability (avoids first-pass metabolism encountered with oral delivery)
- Needle-free (enabling easier self-administration)
- Delivery independent of inspiration (advantageous in some acute settings)
- Dry powder or liquid options available (stability advantages)
- Vaccines can elicit immune response in the area of initial infection (nasal cavity).
One of the most obvious advantages of nasal delivery is the rapid absorption across the nasal membranes into the systemic circulation, resulting in fast onset of action. This means that systemically acting nasal products to be easily and rapidly administered for acute indications. Examples include pain medications for migraines, such as GlaxoSmithKline’s Imitrex®/Imigran® (sumatriptan), Bausch Health’s Migranal® (dihydroergotamine) and Amneal Pharmaceuticals’ Zomig® (zolmitriptan). Generally speaking, drug uptake across the nasal membrane is dictated by the hydrophobicity of the drug (improving transport) and residence time on the membrane.
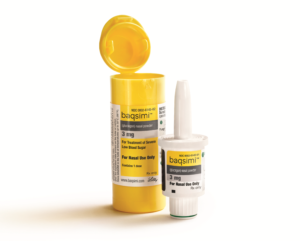
Figure 2: Eli Lilly’s Baqsimi®, a dry powder glucagon nasal spray for the rapid treatment of hypoglycaemia. (Image courtesy Eli Lilly and Company. Reproduced with kind permission.)
In addition to rapid delivery, nasal administration is generally considered more user-friendly, with greater compliance among patients and easier administration for carers and healthcare professionals (HCPs). This route of administration also alleviates the need for the patient to be conscious, uncoupling the need for inspiration from successful delivery, which provides an advantage over pulmonary delivery. These factors have been exploited for the delivery of dry powder glucagon to treat very low blood sugar (hypoglycaemia). Baqsimi®, Eli Lilly’s single-dose, dry powder spray formulation of this rescue treatment, received US FDA approval in 2019 in a portable, single-use, ready-to-use device (Figure 2).
Another major advantage of nasal delivery is that it removes the need for a drug to withstand the harsh environment in the gastrointestinal (GI) tract that arises during oral delivery. As a result, it is possible to avoid first-pass metabolism in the liver, without the need for injection, thereby increasing bioavailability.
A more specialised aspect of nasal delivery that has been under investigation for many years but has recently been enjoying growing interest, is the potential to deliver therapeutic agents directly to the brain. Both the olfactory and trigeminal nerves innervate the nasal cavity, making them a potential target for nose-to-brain delivery. This is a challenging prospect, but next generation devices currently in development may have particular utility in the area of brain disorders such as Parkinson’s disease.
“A more specialised aspect of nasal delivery that has been under investigation for many years but has recently been enjoying growing interest, is the potential to deliver therapeutic agents directly to the brain. Both the olfactory and trigeminal nerves innervate the nasal cavity, making them a potential target for nose-to-brain delivery.”
The final factor currently driving growth in nasal delivery at present is the covid-19 pandemic. The primary route of viral infection is via the upper respiratory tract (i.e. the nose), before increased viral load results in further infection in the lungs. Delivering a range of prophylactics, such as antivirals, and vaccines through the nasal cavity is now considered the best route of delivery for prevention and treatment of this disease.
Nasal vaccination is not new, MedImmue’s FluMist®, approved in 2003, delivers an annual influenza vaccine intranasally and other groups, such as Mymetics (Épalinges, Switzerland), have reported promising results with nasally administered HIV vaccines.
In conclusion, the benefits of nasal delivery have, and continue to be, utilised for a variety of purposes and the growing interest in this area is sure to lead to both new and improved possibilities for nasal administration
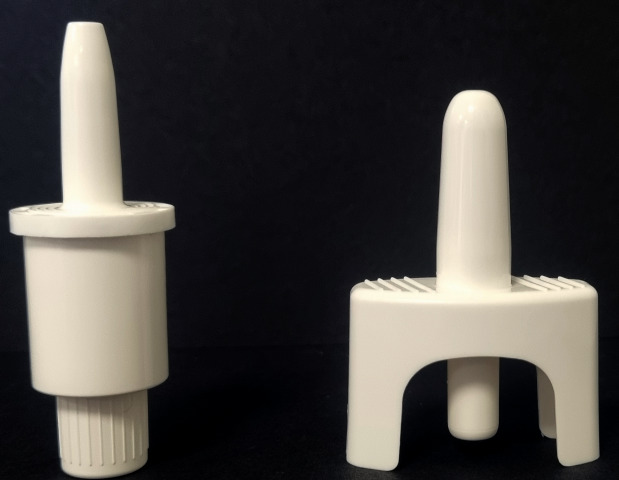
Figure 3: Aptar Pharma’s Unidose (UDS) systems.
LIQUID OR DRY POWDER DELIVERY?
When considering options for nasal delivery of a drug or vaccine, the first decision is usually choice of device type. The options are to deliver the active either in a liquid/solution (as a multidose or single dose unit), or as a dry powder (generally single dose devices), as shown in Figure 3.
Liquid nasal dosage forms are the most prevalent and offer the simplest and most flexible formulation approach. There are a wide range of device options available, which can be purchased relatively inexpensively. The filling of API formulations into liquid devices is easier in comparison with their powder counterparts, and liquid devices also offer the option of either unit-dose or multi-dose delivery. With liquid devices, the droplet size emitted is dictated by the properties of the liquid formulation (viscosity) combined with the performance of the device itself.
In contrast, dry powder devices require specialised formulation techniques, such as spray drying, to engineer the correct particle size required for nasal delivery. This particle size is generally agreed to be in the region of 10–50 μm, with a requirement to minimise small respirable particles (below 10 μm) to avoid the potential of particles being inhaled into the lungs.
Dry powder delivery devices are of growing interest and differ from liquid delivery systems in a number of key areas (Table 1). For example, dry powder formulations are particularly useful in the formulation and delivery of molecules that are relatively unstable in solution but can be stabilised and delivered in the dry powder form, such as peptides and proteins. The delivered dry powder dissolves on the membrane surface of the turbinates, with release of the active ingredients for either local or systemic delivery. However, dry powder devices are generally only single-dose use, and tend to be more expensive than their liquid counterparts.
| Liquid Devices | Dry Powder Devices | |
| Advantages | Simplest, most flexible approach | Improved stability for some formulations |
| Large range of devices available | Fewer solubility constraints | |
| Lower unit cost | Longer nasal residence time | |
| Droplet size device controlled | Particle size engineered prior to device filing |
|
| Disadvantages | Challenge for poorly soluble API’s |
Complex manufacture (particle size) |
| Less stable (aqueous instability of some molecules) | Irritation/discomfort | |
| Shorter nasal residence time | Limited device options | |
| Higher cost |
Table 1: Advantages and disadvantages of dry powder and liquid nasal devices.
FORMULATION DEVELOPMENT: CONSIDERATIONS AND OPTIONS
When developing either a liquid or dry powder formulation, there are challenges and opportunities that can be exploited to achieve the target product profile for the drug or vaccine being delivered. For example, ideally, formulators will want to choose excipients that are listed in the FDA Inactive Ingredients Database for nasally delivered products. However, the list is relatively small and other excipients can be used, although additional toxicology information will be required as part of an IMP/IND/CTD submission.
There are many factors to consider when developing therapeutics and vaccines for nasal delivery, both in regard to optimising the formulations themselves and the devices through which they are delivered. Once the choice between a liquid or dry powder approach has been made, there are a number of particle engineering and excipient options that can be utilised by formulators to achieve the desired product performance attributes needed for successful delivery, release and absorption in the nasal cavity. Some of the formulation considerations are summarised in Table 2, along with suggestions for overcoming the challenges presented.
| Delivery Challenge | Formulation options |
| Achieving required dose delivery into the nose | For liquid devices, formulate API solution (typically 0.1 mL dose will be delivered) For dry powder devices, formulating API into correct dry powder mass is important (note there may be constraints on mass of powder that can be delivered from the device; typically 10–20 mg) |
| Targeting the nasal membranes |
Liquid formulation and devices, properties adjusted to achieve target droplet size, in particular the use of viscosity enhancements (e.g. HPMC, PVP) to achieve correct droplet size For dry powder devices, it is necessary to engineer correct particle size in powder formulation prior to filling device (typically 20–50 μm) Spray drying is often used to produce particles of correct size |
| Addressing solubility | For liquid formulations, solubility can be achieved by pH adjustment, salt formation or use of non-aqueous solvents (e.g. ethanol, cyclodextrins) For dry powder devices this is less of an issue, formulation of the API and production of particles is achieved prior to device filling |
| Achieving pH control | Average value for pH reported as 6.3–6.4 Many nasal products have lower pH e.g. Narcan® (naloxone) pH 3.5–5.5 |
| Achieving osmolality | Isotonic solution (~290 mOsmol/kg) will minimise irritancy/maximise tolerability Achieved by adding NaCl or sugars for osmolality upwards adjustment |
| Avoiding microbial growth in final dosage form | Multidose products have preservatives (e.g. benzalkonium chloride) but single dose (liquid) devices are usually preservative-free Not an issue with dry powder devices (no growth) |
| Adsorption modulation | Potential to slow down the rate of absorption Add viscous polymers (e.g. cellulouses, pectin) Applicable to liquid and dry powder devices |
| Adsorption enhancement | Chemical enhancement of drug uptake by adding enhancers such as dodecylphosphocholine, (zwitterionic detergent used in Baqsimi®) or chitosan (cationic polymer that binds to mucus) Applicable to liquid and dry powder devices |
Table 2: Delivery considerations and formulation options.
TESTING LIQUID AND DRY POWDER DEVICES
Similar to the choice of device and formulation type, the choice of analytical methods will be very different for the liquid and dry powder dosage forms, and depend greatly on the stage of development and regulatory territory. Broadly speaking, the required analysis will include:
- Liquid dosage form:
– Comprehensive testing of the liquid in the device, including the API and key excipients
– Droplet size
– Plume geometry
– Spray pattern
– Quantity of liquid/drug delivered. - Dry powder dosage form:
– Comprehensive testing of the dry powder drug product within the device
– Particle size
– Plume geometry
– Quantity of dry powder delivered from the device.
DEVELOPMENT STUDIES
During development of nasal products, a wide range of performance attribute tests are recommended and/or stipulated by the regulatory authorities in addition to the more typical expected specified final product release testing. Both the EMA and FDA provide detail on a lot of these tests, which are often dependent on both the formulation and the device and can be used to discriminate between formulation variants. These tests include:
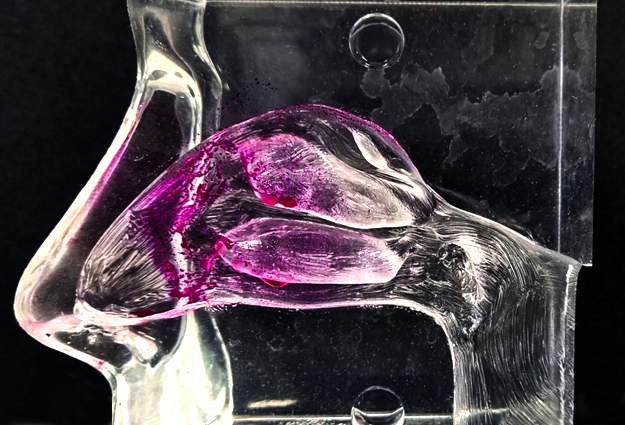
Figure 4: Transparent nasal cast coated with water-reacting paste. Formulation fired into cast with multidose liquid device.
- Delivered dose throughout the life of the product
- Priming requirements
- Effect of dosing orientation on emitted dose
- Plume geometry and spray pattern.
Another tool that has proven useful in comparing and selecting formulations during development is the transparent nasal cavity (Figure 4), which can visualise the effect of formulation changes, the delivery device and changes in orientation during use. The cavity is coated such that a colour change occurs when it comes into contact with the formulation delivered from the nasal device. By examination of the colour, the specific areas of deposition in the nasal cavity can be identified and key performance failure modes can be observed, such as excessive formulation run-off to the throat or excessive deposition in the nasal vestibule resulting in the formulation “dripping out”.
| Assay type | Comment |
| Spray characteristics | Solutions (droplets size, plume geometry) usually for information only |
| For dry powders will include particle size determination (specify small/inhalable particles) | |
| Assay/delivered dose | Should correlate/verify any weight method with drug capture |
| Quantification (API and functional excipients) | Assay (e.g. preservatives) |
| Physical measurements (viscosity) |
Table 3: Testing nasally delivered drug products for use in Phase I studies.
TESTING CONSIDERATIONS FOR PHASE I STUDIES
The exhaustive list of tests needed for commercial products are generally not required for Phase I (first-in-human) studies. Instead, a more pragmatic approach can be taken. Table 3 shows typical assays that are suitable for supporting Phase I studies.
| Test | FDA | EMA | Comments |
| Description | √ | ||
| Identification | √ | ||
| Assay | √ | √ | API content and stability |
| Impurities/related substances | √ | √ | API content and stability |
| Preservatives | √ | Confirm levels and stability of preservative | |
| Pump delivery | √ | ||
| Spray content uniformity | √ | √ | Solutions measured by weight difference. Powder devices; collect in container and assay for active |
| Spray pattern | √ | Laser based system or TLC plate analysis | |
| Droplet/particle size distribution |
√ | Laser diffraction (e.g. Malvern Panalytical’s Spraytec) for liquids or Andersen type impactor/laser diffraction for dry powders | |
| Plume geometry | √ | Video/photographic imaging at specified distance |
|
| Particulate matter | √ | Sub-visible particle analysis | |
| Microbial limits | √ | √ | Confirm levels and stability of preservative (if present) |
| Net content | √ | ||
| Weight loss | √ | ||
| Extractable/leachables | √ | √ | Required for plastic devices (FDA) Not required by EMA for compendial plastics |
| pH | √ | Typical values would be in the pH 5.0–8.0 range |
|
| Osmolality | √ | Isotonic solution (~290 mOsmol/kg) will minimise irritation |
|
| Viscosity | √ | Impacts droplet size and plume geometry |
Table 4: Product specifications for nasal drug products.
TESTING NASAL FORMULATIONS: REGULATORY REQUIREMENTS
The degree of testing necessary will be dictated by the regulatory setting and the stage of development of the drug/device combination. Typical testing regimens for liquid and dry powder nasal products are shown in Table 4. As can be seen, there is an extensive range of tests required when developing nasal dosage forms for human use. Some of these tests require sophisticated pieces of equipment, many of which might only be found in laboratories that have established nasal testing capabilities. The range and extent of testing will primarily be dictated by the development stage of the formulation to be tested. In simple terms, products earmarked for use in Phase I clinical testing will require significantly less testing than products being tested for commercial use. In Phase I studies, the key tests will be those that impact product safety, such as particle size/droplet size (impact on pulmonary toxicity) and emitted dose. These tests will provide key safety and delivery information to support use in first in human studies. As the drug product moves further through the development process, the extent of testing will be required to increase to allow the drug to be sold and used commercially and more stringent tests, such as device compatibility (extractables/leachables), will become necessary.
CONCLUSION
This article has outlined the logistics and considerations associated with developing a drug or vaccine for nasal delivery. It has discussed the advantages the anatomy of the nasal cavity provides for drug delivery, the challenges of developing both liquid and dry powder formulations, analytical techniques and regulatory testing requirements.

