Citation: Blouet E, “A New Multi-Compendia Modified Beta-Cyclodextrin, KLEPTOSE® HPB-LB Parenteral Grade”. ONdrugDelivery Magazine, Issue 97 (May 2019), pp 12-15.
Elham Blouet introduces the latest addition to the company’s range of beta-cyclodextrins, and outlines the benefits it brings.
Roquette, a leader in beta-cyclodextrins (KLEPTOSE®), recently expanded its range by launching KLEPTOSE® HPB-LB – a new grade of modified cyclodextrin: hydroxypropyl-beta cyclodextrin (HPβCD) as an excipient grade for use in parenteral applications. Meeting the highest global purity standards and following the principles of GMP, KLEPTOSE® HPB-LB parenteral grade will facilitate the registration of pharmaceutical products in multiple target markets.
“It is the ability to form inclusion compounds through molecular
encapsulation that gives HPßCD its interest as a formulation aid.”
Increased interest in cyclodextrins (CDs) in recent years has led to a strong market demand, and several new pharmaceutical products containing beta-cyclodextrins or their derivatives have reached the market succesisfully. To meet the specific needs of the pharmaceutical industry, Roquette now offers a wide range of KLEPTOSE® products: beta-cyclodextrins and HPβCDs.

Figure 1: Chemical structure of beta-cyclodextrin.
ONE SOLUTION FOR GLOBAL COMPLIANCE
KLEPTOSE® HPB-LB parenteral grade is a multi-compendia product that complies with the European Pharmacopoeia (EP) and US Pharmacopeia (USP) – and has standards that not only comply with but are even higher than those of the Chinese pharmacopoeia. Part of the wider KLEPTOSE® product range, KLEPTOSE® HPB-LB supports local and global pharmaceutical manufacturers in overcoming registration filing challenges in China, as well as the rest of the world, without the need to develop multiple drug solutions. This can accelerate speed to market and provide a competitive advantage.
A VERSATILE EXCIPIENT
Both native and modified CDs have the ability to form inclusion compounds through molecular encapsulation with a wide range of organic molecules. This ability makes CDs and their derivatives valuable as formulation aids.
They are used to increase the aqueous solubility of poorly soluble drugs and so avoid the use of organic solvents. Their use is also of great interest for improving the physical and chemical stability of drugs (protection against light, oxidation, etc.), for enhancing local tolerance of drugs andfor any other applications where inclusion compounds would enable innovative solutions. Oral, parenteral, topical and ophthalmic preparations containing CDs and their derivatives are marketed worldwide.
Therefore the new KLEPTOSE® HPB-LB, parenteral grade is expected to improve active substance stabilisation against light and oxidation in parenteral preparations, and can also be used as a solubility enhancer.

Figure 2: Chemical structure of hydroxypropyl-beta-cyclodextrin.
UNIQUE MOLECULAR STRUCTURE
CDs are cyclic oligosaccharides (Figure 1) obtained from starch by enzymatic cyclisation using cycloglycosyltransferases. They are composed of α-(1.4) linked glucopyranose subunits. The beta-cyclodextrin, composed of 7 α-(1.4) glucopyranose units, is the most accessible and useful one with significant industrial usage. Roquette has branded its beta-cyclodextrin as KLEPTOSE®. The HPβCD is a CD chemically modified by hydroxypropylation. HPβCDs are purified polydisperse products resulting from the controlled reaction of propylene oxide and native beta-cyclodextrins under base catalysis. HPβCD has the highest aqueous solubility (65% at 25°C) and, combined with its safety profile, it represents an ideal profile for pharmaceutical applications. Thanks to its safety profile, the HPβCD is a suitable excipient for parenteral applications as well as for oral, topical and ophthalmic applications.
The HPβCD molecule is a torus-shaped ring with a polar hydrophilic exterior and an apolar hydrophobic cavity. This structural feature is due to the spatial distribution of its external hydrophilic properties. As a result of this structure (Figure 2), HPβCD encapsulates or entraps guest molecules to form so-called inclusion compounds when in an aqueous solution.
The secondary OH groups on C-2 and C-3 are on the opposite edge, which gives HPβCD its external hydrophilic properties. The inside of the HPβCD ring is composed of a surface of hydrophobic C-3 and C-5 hydrogens as well as glycosidic ether-like oxygen.
The molar substitution (MS) is the average number of hydroxypropyl groups per anhydroglucose unit.
The degree of substitution (DS) is the number of hydroxypropyl groups per molecule of HPβCD and is obtained by multiplying the MS by seven. KLEPTOSE® HPB-LB is a composite product with a specific substitution pattern. The consistency of this substitution pattern is guaranteed by the manufacturing conditions applied by Roquette. The MS range of KLEPTOSE® HPB-LB complies with the EP/USP requirement (0.40-1.50) and ChP requirement (0.50-0.71.)
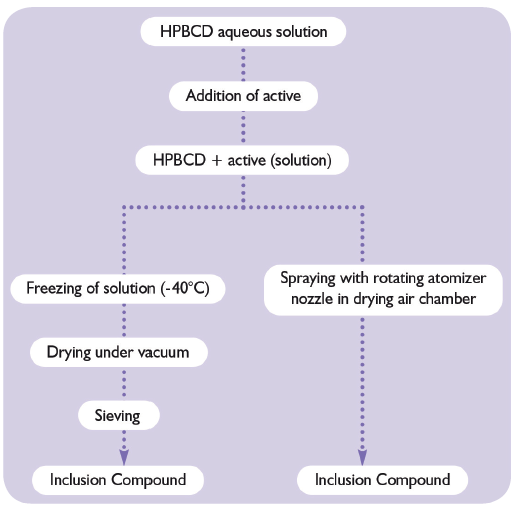
Figure 3: Preparation of inclusion complex in aqueous solution.
HPßCD INCLUSION COMPLEXES
With HPβCD, the preparation of inclusion compounds or complexes in aqueous media is very simple. The general principle involves the solubilisation of the predetermined amount of HPβCD. An instant aqueous solution is obtained. The active ingredient is added to this solution and mixed until a clear solution is formed. Ultimately, the complex can be freeze dried or spray dried (Figure 3).Other more sophisticated techniques such as supercritical CO2 exist. For initial trial purposes, and to determine the right amount of HPβCD to be used, the following protocol can be applied: add the active ingredient to a 50% HPβCD solution until a precipitate is formed.
It is the ability to form inclusion compounds through molecular encapsulation that gives HPβCD its interest as a formulation aid. Molecular encapsulation between HPβCD and a guest molecule is an equilibrium reaction (no covalent bonds) characterised by a binding constant (Kc) which is specific to each guest – HPβCD complex (Figure 4). In practical terms, the higher the binding constant, the higher the affinity of the guest molecule for the HPβCD.
The ability of a guest molecule to form a complex with an HPβCD molecule is a function of two main factors:
- Steric factor (size and shape of the guest molecule), which explains that a molecule can be partially or totally encapsulated
- Thermodynamic interactions between the different components.
Molecular encapsulation, like any other chemical reaction, is ruled by thermodynamic laws. Consequently, the addition of formulation additives may influence the inclusion either positively through the formation of ternary complexes (e.g. with aqueous soluble polymers, organic hydroxy acids or certain organic bases) or negatively because of competition with the guest molecule (e.g. with bile salts). Moreover, an energy input through temperature increase or operations (shear, pressure) can increase the complexation efficiency (Figure 5).
The release of the guest molecule from the HPβCD complex is driven by two main factors:
- The dilution effect
- Competition with other molecules which have a higher affinity for HPβCD complexation.
Other factors can influence complex formation. Interaction between formulation ingredients is of particular importance and must be evaluated. For instance, thiomersal and benzylic alcohol can be recommended as preservatives because they do not compete with the guest.
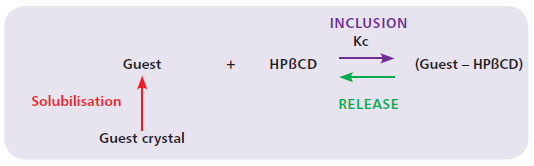
Figure 4: Inclusion complex equilibrium reaction.
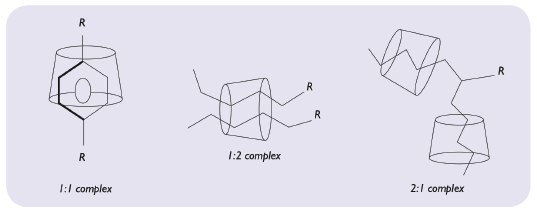
Figure 5: Representation of molecular encapsulation possibilities.
PARENTERAL APPLICATIONS
HPβCD is an attractive excipient in injectable dosage forms as it is highly water soluble and with high biological tolerance.
The main functionality of HPβCDs are:
- Enhancing solubility of poorly soluble active substances to improve their bioavailability
- Stabilising active substances against oxidation, hydrolysis, heat degradation and light degradation
- Reducing irritation at the injection site while having low toxicity.
One of the reasons for using the injection route of administration is for a systemic fast-acting result, which is why the drug must not only be more soluble but also dissolve more quickly. There are numerous publications on the solubilising power of HPβCD; the examples given here are for illustration only: the effect of HPβCD on the solubility of some drugs of interest in injectable application is shown in Table 1.
| Carbamazepine | Danazol | Albendazole | ||||
| HPβCD (mM) |
Solubility mg/mL |
S/SO mg/mL |
Solubility mg/mL |
S/SO mg/mL |
Solubility μg/mL |
S/SO μg/mL |
| 0 | 0.097 | 1 | 1.42×10-4 | 1 | 1.254 | 1 |
| 10 | 0.788 | 8 | 0.193 | 1362 | 20.181 | 16 |
| 20 | 1.45 | 14 | 0.34 | 2396 | 37.178 | 29 |
| 30 | 2.197 | 22 | 0.523 | 3684 | 46.806 | 37 |
| 40 | 3.107 | 31 | 0.774 | 5451 | 70.376 | 56 |
| 50 | 3.927 | 40 | 0.94 | 6623 | 74.153 | 59 |
| 100 | 6.723 | 69 | 1.983 | 13965 | 146.353 | 116 |
| 200 | 11.805 | 121 | 4.239 | 29854 | 352.701 | 281 |
SO is the drug solubility in DI water
Table 1: Solubility increase as a function of HPßCD molarity.1
VALUE-ADDED BENEFITS
The new KLEPTOSE® HPB-LB, (HPβCD), parenteral grade presents multiple benefits with regards to regulatory compliance, physical and chemical properties, performance, quality systems and enhanced packaging:
- Multi-compendia, enabling access to the global market
- High water solubility (ideal for small volume parenterals)
- Low viscosity: 20 cP at 20°C, and 40% HPβCD: ideal for injection, especially subcutaneous
- Endotoxin controlled, making it suitable for parenteral applications
- Encapsulation process versatility
- Encapsulation efficiency of a wide range of molecules
- Stability at high temperature allowing terminal steam sterilisation
- Stability at hydrolysis over a wide range of pH
- Production and quality systems following GMP principles
- Fibre-free packaging, with tamper-proof evidence
- Enhanced packaging with recyclable materials.
ROQUETTE RANGE OF MODIFIED KLEPTOSE®
Roquette has a full range of modified HPβCD. The key points of each grade of KLEPTOSE® HPβCD are listed in Table 2.
| KLEPTOSE® HPB, parenteral grade | KLEPTOSE® HP, parenteral grade |
KLEPTOSE® HPB , oral grade | KLEPTOSE® HP, oral grade |
KLEPTOSE® HPB, Biopharma | KLEPTOSE®, HP Biopharma |
KLEPTOSE® HPB-LB, parenteral grade |
|
| Grade | Parenteral | Parenteral | Oral | Oral | Biopharma (low endotoxins) |
Biopharma (low endotoxins) |
Parenteral |
| Molar Substitution (MS) | 0.58 – 0.68 | 0.81 – 0.99 | 0.58 – 0.68 | 0.81 – 0.99 | 0.58 – 0.68 | 0.81 – 0.99 | 0.50 – 0.71 |
| Applications | Small molecule | Small molecule | Small molecule | Small molecule | Large molecule | Large molecule | Small molecule |
| Route of administration | Parenteral, ophthalmic and topical |
Parenteral, ophthalmic and topical |
Oral and topical | Oral and topical | Parenteral | Parenteral | Parenteral, ophthalmic and topical |
| Regulatory compliance |
EP / USP NF | EP / USP NF | EP / USP NF | EP / USP NF | EP / USP NF | EP / USP NF | EP/ USP NF / ChP |
| CEP | Yes | Yes | No | No | No | No | No |
| DMF | US DMF (type II & IV) |
US DMF (type II & IV) |
US DMF (type IV) |
US DMF (type IV) |
US DMF (type IV) |
US DMF (type IV) |
Chinese DMF |
Table 2: Key points of the KLEPTOSE® range of hydroxypropyl beta-cyclodextrins (HPßCDs).
CONCLUSION
For more than 40 years, Roquette has made patient safety, improving health and ensuring formulation safety among its top priorities. As a pioneer in pyrogen-free pharmaceutical ingredients, Roquette has set the standard for highly purified excipients and APIs – enabling formulation with confidence. With multiple manufacturing sites across the world, supported by a vertically integrated supply chain, Roquette provides the confidence needed to develop safe and efficacious pharmaceutical products.
As an innovator in the industrial development of cyclodextrins with its KLEPTOSE® range of beta-cyclodextrins, Roquette proudly introduces its new KLEPTOSE® HPB-LB parenteral grade product to the portfolio. As a trusted partner and leading integrated supplier offering full traceability and supply chain security, your pharmaceutical applications will meet the highest quality and regulatory requirements because Roquette is committed to securing the purest ingredients for use in reliable oral and parenteral preparations to customers and future customers globally.
REFERENCES
- Popescu C et al, “Determination of the Thermodynamic Solubility and the Affinity (Binding) Constants of Carbamazepine, Danazol and Albendazole in Hydroxypropyl Beta Cyclodextrin (KLEPTOSE®HPB) Solutions”. AAPS Annual Meeting, 2011.


