To Issue 145
Citation: Cleall A, Smith B, “Are you Struggling to Put Difficult Powders into Capsules?”. ONdrugDelivery, Issue 145 (Apr 2023), pp 39–42.
Andy Cleall and Ben Smith discuss process optimisation and cutting-edge technology for powder filling, including how 3P Innovation’s R500 and R1000 robotic capsule fillers are pushing the boundaries of optimal filling and how its Fill2Weight technology enables increased filling accuracy and improved yields.
“Lower fill weights are becoming increasingly important as the pharmaceutical industry continues to consider drivers such as modularity, agility and the development of personalised medicines.”
THE NEED FOR ADVANCED FILLING TECHNOLOGY
With the pharmaceutical industry under increasing pressure to develop novel drug products faster, it is important that manufacturers make use of the most advanced technologies. This is particularly true for capsule filling; drug developers often only have small-but-expensive amounts of test material to work with, so cost-efficiencies and time-to-market are critical.
3P innovation’s R500 and R1000 are robotic capsule fillers (Figure 1). These machines were designed to provide a low-cost and flexible way to produce capsules. They can also be configured to fill certain delivery devices and containers, including vials, syringes and cartridges. One of the key issues concerning 3P’s pharma customers is the difficulty associated with low-dose fill weights, particularly when they’re looking at fill weights of 1.0 mg or less. By investigating this, 3P has generated quite a bit of data in terms of filling 0.5–1.0 mg of pure API into capsules.

Figure 1: 3P innovation’s range of capsule fillers.
The challenges manufacturers face when dealing with low fill weights include weighing accuracy, controlling static and regulating air movement, all while operating an automated process with an expected output of more than 200–300 capsules an hour. Furthermore, this needs to be achieved within the context of a fill-to-weight environment of chronicling 100% weight records.
Lower fill weights are becoming increasingly important as the pharmaceutical industry continues to consider drivers such as modularity, agility and the development of personalised medicines. 3P manufactures machines that are part of the production of current advanced therapies, and the company is fielding an increasing number of enquiries about using this filling technology.

Figure 2: Ben Smith in front of an R1000.
R1000 AND R500
The R1000 and R500 are versatile and powerful production machines (Figure 2), capable of handling the most challenging and specialist powders – from pure API to blends, delicate spray-dried powders and micropellets. Featuring the ground-breaking Fill2Weight (F2W) powder-filling technology, these systems can handle a wide range of powders and deliver precise microdosing under adverse conditions, such as low relative humidity and containment. As such, they are able to handle pure and highly potent APIs.
One of the benefits of the R500/R1000 range and F2W solutions is that they can use the same set of change parts. Without having to make any physical replacements or swap-outs, a user can change its target dose weight with the machine’s software. This is a considerable advantage, as users can switch from a 2 mg to a 5 mg fill, or from a 10 mg to a 100 mg fill, with no part changeovers required at all.
The technology is also compatible with both free-flowing and highly cohesive powder types, and can handle either with the same reusable change parts. For example, with clinical trial batch dose sizing, this flexibility offers significant benefits in terms of reducing the complexity of the trial set-up. By compressing clinical development timescales and reducing costs, this highly flexible system can fill challenging powders without having to change the physical hardware. Even better, there is no minimum batch size.
Adding to that, 3P’s customers are more frequently dealing with very difficult-to-handle powders, such as those with bulk densities down in the 0.2 g/cm3 range. These powders tend to be highly cohesive and difficult to fill using conventional technologies. By applying low amplitude, high frequency vibration at the nozzle, the cohesive bonds are overcome and van der Waals forces are disrupted enough to fluidise the powder to enable filling. Simply put, if the right parameters are not applied, the powder will not come out, but, with the right amount of Hertz and a certain amplitude, 3P has been able to mobilise some unbelievably difficult powders that would most likely choke a micro- or vertical auger.
“To improve yield, 3P developed a ‘top-up’ algorithm to correct slightly underweight doses.”
3P’S IN-HOUSE TESTING
3P can run in-house trials for its customers with its F2W system, including for difficult powders, to optimise their recipe settings. This is important – if a drug developer is preparing to make a clinical batch, they do not always know the physical properties of the powder; therefore, they may not know what technologies to invest in. By the time the developer gets the powder, they need to have the appropriate technology ready; when companies put that powder through the machine for the first time, they cannot afford to spend time reformulating the recipe to make it work.
Test batches generally comprise 5–10 g of expensive micronised or spray-dried material and, not only do drug developers want results quickly, they want to know that their powder flows well enough to fill capsules with anything up to 200 mg. 3P provides the confidence that the customer can return to their machine, apply the same settings and achieve that goal. What 3P is doing is enabling and de-risking their customers’ clinical trial programmes by reducing the number of formulation steps involved and getting to the first-in-human stage quicker.
F2W TECHNOLOGY IN PRACTICE
One material 3P has been working with is a spray-dried particle that is being used as an immunotherapy. Part of the spray-dried formulation is a polymer, which gives the mix a certain plasticity, similar to polystyrene balls at a macroscopic scale. The inherent issue with these highly charged, static-sensitive powders is that they are difficult to dose. In fact, it is often more of a problem getting the powders into the equipment than it is getting them out; they tend to be cohesive and need gentle handling, which the R500 can accommodate (Figure 3).
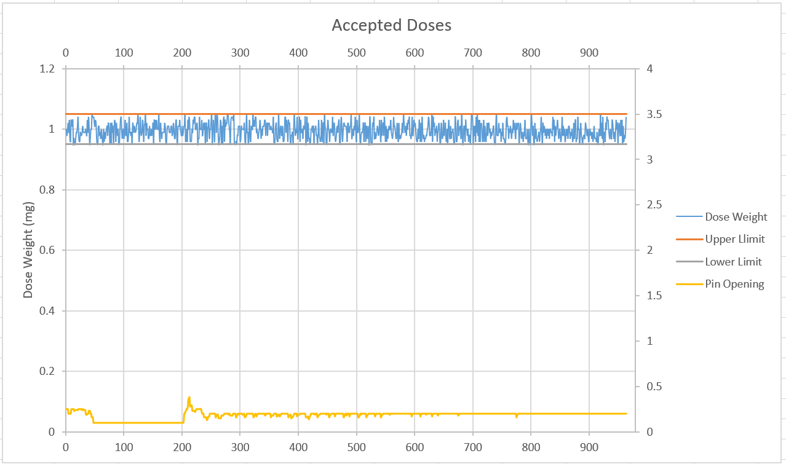
Figure 3: Dosing 1.0 mg ± 0.05 mg of a cohesive, spray-dried particle using 3P’s F2W technology.
Getting difficult powders into a container with a long and/or narrow opening, such as a syringe, is something 3P has quite a lot of experience with. Typically, 3P will pass the nozzle tube into the device to place the dose at the base of the container and minimise particle adhesion to the container walls. The aim is to avoid any further need for reformulation and enable the customer to use the powder that they have already produced. From the starting point of a good working recipe, all that is required is to decide on the new dose weight and adjust the settings for a larger or smaller clinical trial accordingly, based on the initial settings, without having to reformulate to achieve a specific bulk density or needing access to a particular piece of equipment.
When attempting a deep fill under uni-directional airflow conditions, particularly when working with plastic containers, electrostatic effects can be problematic. At the same time, it is critical not to contaminate the side walls. This is something 3P does routinely – when filling syringes, 3P places the powder into the syringe barrel via an extended nozzle tube, which is vibrated at a high frequency to fluidise the powder, stop it sticking and complete the fill as quickly as possible.
Real-time F2W works very well here; as the powder lands, its weight accumulates and the system plots the increase in weight, calculates the mass flow rate and then works out when to stop. The technology does this repeatedly throughout the dose at more than 50 times per second. For a larger dose, it is typical to bulk dose up to two-thirds of the powder in very quickly and taper the flow rate until the target weight is achieved; a low dose weight, such as 1–10 mg, would be done in a single operation.
When working with very fine powders and very low doses, a few particles might clump together as they leave the nozzle. And, because the technology is weighing in divisions of hundredths of a milligram, the weight changes in a stepwise manner. So, as the machine approaches the target, it might interpret one of those increases in flow rate and make an adjustment to ensure an accurate fill, with the margin of error always erring on the side of underfilling rather than overfilling.
In the past, to make 1.0 mg work for a customer, 3P sought to achieve a a balance between yield and rejection rates. As long as a certain percentage of the capsules, say 60%–70%, had the required fill, customers were happy to accept that ratio – it is one of the compromises required when working with very low dose weights. So, to improve yield, 3P developed a “top-up” algorithm to correct slightly underweight doses. This top-up feature provides an excellent way to improve yields; when the fill volume is just underneath the target, the system now recognises what has happened and, instead of abandoning the dose, it goes back, has another go and adds a small amount of powder to reach the prescribed target. 3P’s data show that this achieves very high yields (95%–100%) for more than 1,000 capsules.
Another area of application in which F2W shows great promise is that of inhaled and nasally delivered therapies. These dosage forms are often quite friable and, given the need to be aerosolised, the particles cannot clump together. Likewise, they cannot be compacted or compressed for delivery to the lungs. As such, it is often necessary to work with low-volume, concentrated doses of pure APIs, which makes dosing accuracy even more important.1 F2W technology is a non-compacting, low-shear process that overcomes many of these issues. Furthermore, an ioniser can be placed in the head to ionise the powder during feeding to minimise the static attraction between particles.

Figure 4: R1000 robotic capsule filler.
CONCLUSION
In summary, the upgraded weigh cell in 3P’s R1000 and R500 features a display resolution of 0.01 mg, retaining high-speed feedback for gravimetric filling precision control (Figure 4). It will weigh powder aliquots of less than 1 mg with a 1% tolerance during production to comply with USP <41> guidelines. In addition, the enhanced robot-controlled operation ensures a smooth filling process for weight stability at low dose weights. Finally, the upgraded F2W software provides additional controls with automated “top-up” functionality for higher yields and accuracy. These cutting-edge machines enable users to develop and launch new drugs to market quicker, reducing clinical development timescales and achieving first-in-human milestones faster and at lower costs.
REFERENCE
- French T, “Breaching the Blood-Brain-Barrier with Particle- Based Nasal Delivery Systems”. ONdrugDelivery, Issue 131 (Apr 2022), pp 27–30.


