To Issue 145
Citation: Kobler M, Sule A, Sule S, “Development Approach for a High-Performance Capsule-Based DPI”. ONdrugDelivery, Issue 145 (Apr 2023), pp 44–47.
Mirjam Kobler, Ameet Sule and Sunita Sule look at the factors affecting the performance of dry powder inhalers and discuss H&T Presspart’s development of high-performance prototypes.
NEW APPLICATIONS – THE NEED FOR HIGH PERFORMANCE
The pulmonary route for drug delivery is becoming increasingly attractive to drug developers, not only for low-dose locally acting therapies, such as asthma and chronic obstructive pulmonary disease (COPD), but also for systemic applications that often require higher doses or novel formulation technologies.
“In many cases, capsule-based inhalers are the preferred solution for new applications because they offer several interesting features.”
High-performance devices must be developed to accommodate the requirements for delivering these new formulations efficiently. To ensure the best performance, the development of the formulation and the device should go hand in hand.
Dry powder inhalers (DPIs) are used to deliver medications to the lungs in powder form via oral inhalation. Medication in powder form can be filled in either a capsule, blister, reservoir or cartridge, depending on the drug product configuration. The device can be either an active or a passive device. In many cases, capsule-based inhalers are the preferred solution for new applications because they offer several interesting features, such as the possibility to use a wide range of different APIs and doses, generally ranging from 5 to 50 mg. Further advantages include ease of use and a good feedback mechanism for the patient.
Due to the complexity and cost of manufacturing multidose DPIs, pharma companies try to minimise costs and risks in new applications by using simpler and more affordable devices.
For some new applications, such as pain relievers or antibiotics, a reusable device is beneficial; for others, such as vaccines, a disposable single-use device is preferable. In both cases, capsule-based inhalers can be a good solution.
The covid-19 pandemic has made it imperative that an easy-to-use vaccine is available worldwide and remains stable under ambient conditions. Research continues to focus on delivering the vaccine via the inhalation route.1
However, there are some drawbacks associated with current capsule-based DPIs, such as low performance, which is an inherent feature of many capsule-based DPIs on the market. Even newly developed integrated solutions for antibiotics, such as tobramycin, do not reach a fine particle fraction (FPF) higher than 35%. To achieve the required therapeutic dose of the antibiotic, the patient must inhale four capsules a day.2
Many strategies for overcoming the inherent challenges of most existing devices are achieved by focusing on improving the dispersion properties of the formulations. Not much attention has been paid to improving the devices, either by technological advancements in device performance (higher de-agglomeration and less retention of powder in the device/ capsule) or on increasing patient adherence for better handling, and thus higher deposition in the deep lungs.3
The novel H&T Presspart DPI addresses the above problems and challenges of capsule-based inhalers. The most important features of an ideal capsule-based DPI are ease of use with a good feedback mechanism for the patient coupled with a cost effective design. In addition, dose delivery with high reliability and consistency, and high-performance efficiencies for a wide range of applications are desirable.
DEVICE ENGINE AND DEVELOPMENT APPROACH
While developing a novel capsule-based inhaler, the main focus was to create a high-efficiency engine to de-aggregate and aerosolise the powder formulation in a medium resistance and relatively flow-rate independent device. Several prototypes were tested and studied throughout the development journey.
The airflow through the capsule chamber was designed so that the capsule oscillates. This causes impaction of the capsule within the capsule chamber, leading to the breakdown of big powder aggregates inside the capsule and, consequently, an efficient release of the powder from the capsule.
After the initial de-aggregation of the powder in the capsule, the powder evacuates from the capsule and reaches the swirl chamber. Here, further de-aggregation takes place due to the shear forces caused by the turbulent airflow and the impaction of the powder on the walls of the swirl chamber. The combination of the capsule movement and powder flow in the swirl chamber leads to a highly dispersed powder. This powder exits into the tubular mouthpiece through the mesh.
The powder de-aggregation and dispersion potential of a device are critical parameters for achieving high efficiency. Various techniques can be used to study airflow and powder de-aggregation behaviour within a device. Here, a combination of simulation with computational fluid dynamics (CFD) modelling in a steady state and an experimental approach were used to develop the new device.
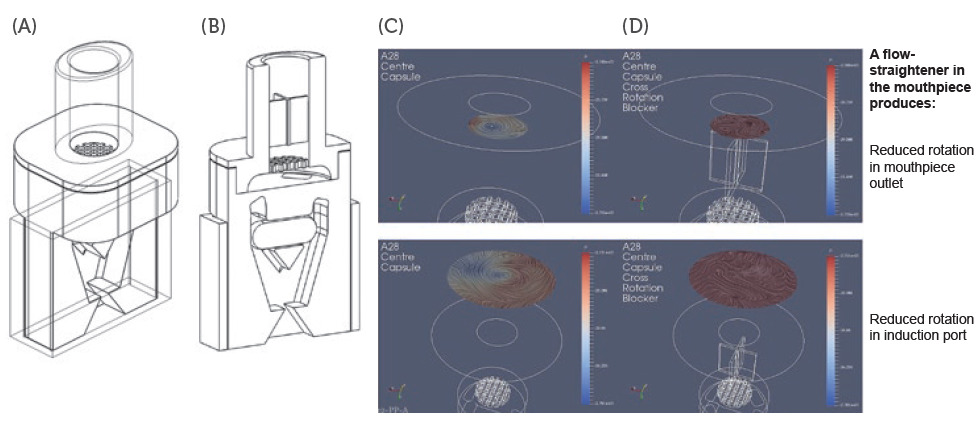
Figure 1: Schematics of DPI prototypes: Prototype 1 without flow straightener (A) and Prototype 2 with flow straightener (B) configuration. CFD simulations for Prototype 1 (C) and Prototype 2 configuration (D).
In one experimental design, CFD models were used to study the airflow structures within the device. Two prototypes were tested, Prototype 1 (Figure 1A), without a flow straightener, and Prototype 2 (Figure 1B), with an integrated flow straightener in the mouthpiece. A fine particle assessment using a next-generation impactor (NGI) was conducted on the prototypes. As seen in Figure 1C, the CFD data of Prototype 1 exhibits a swirling flow that proceeds out of the mouthpiece, reflecting a higher deposition and a swirl pattern observed in the induction port of the NGI. However, with the introduction of the flow straightener in Prototype 2, the CFD data shows a high reduction in the swirl exiting the mouthpiece (Figure 1D).
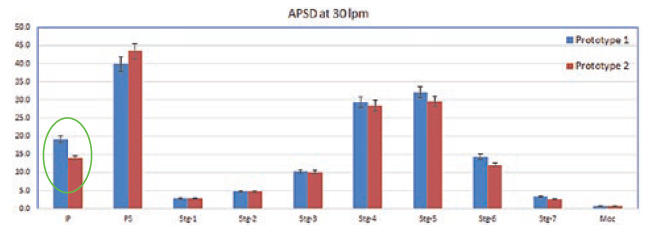
Figure 2: Comparison of NGI deposition of Prototype 1 and Prototype 2 at 30 L/min.
A comparison of the CFD data also shows a correlation with the NGI data (Figure 2). There was a higher deposition in the induction port at both the flow rates for Prototype 1, which was significantly higher at 30 L/min (P < 0.05). The statistical analysis performed using an independent student t-test gave probability values of < 0.05, which was considered to be a significant difference. The flow straightener reduced the swirl of the airflow exiting the device, thereby reducing the deposition in the induction port. There was no significant difference in FPF between the two devices indicating that major de-aggregation occurs within the device. CFD proved to be a valuable tool for studying the air flow dynamics of the DPI. NGI testing provided the supporting data and visual observation of the drug deposition in the induction port, which indicated probable oropharyngeal deposition.
A modular engine set-up during development allowed for easily making small changes in the prototypes. Significant improvements could be achieved by changing different engine parameters. Figure 3 demonstrates the improvement in performance of the test data from Prototype 1 to 4. The NGI testing demonstrated a reduction in the deposition in the induction port and pre-separator stage, as well as an increase in the lower stages of the impactor, which resulted in an increase in FPF as design development progressed.
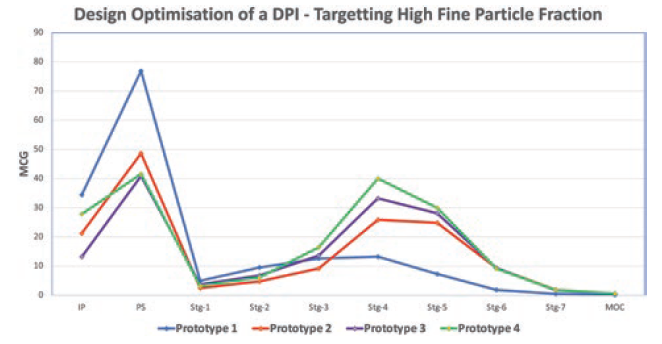
Figure 3: NGI deposition profile of a salbutamol sulfate carrier-based formulation with different prototypes of the Presspart capsule-based device.
“The inspiratory resistance of the new H&T Presspart device was designed as a medium resistance device.”
RESISTANCE
The intrinsic resistance of a device is often discussed controversially in the literature. For asthma and COPD applications, low-resistance devices often seem to be more beneficial for the patient struggling to achieve high flow rates. However, low device resistances often come with a lower deposition of fine particles of the API in the deep lung. Medium resistances have the big advantage of increasing deep lung deposition.4 The inspiratory resistance of the new H&T Presspart device was designed as a medium resistance device.
Another development target was to achieve a relatively flow-rate-independent device. The airflow path was designed and optimised such that there was no significant difference in the FPF for flow rates ranging from 30 L/min to 90 L/min (Figure 4).
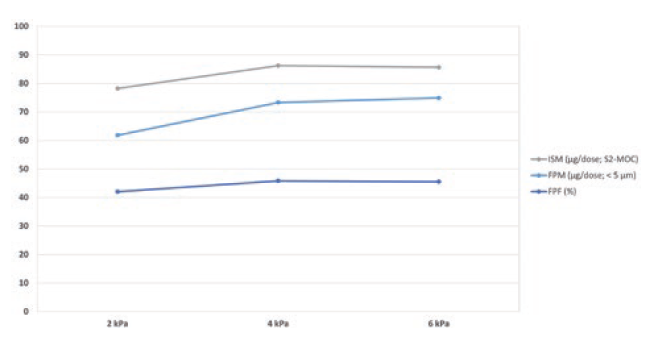
Figure 4: Fine particle delivery of budesonide 200 μg powder for inhalation at 2, 4 and 6 kPa.
“The FPFs generated were highest for the H&T Presspart device when compared with the commercially available standard devices HandiHaler and RS01 equivalent.”
EVALUATION OF DIFFERENT FORMULATION TYPES
Two types of formulations were studied: a binary mixture and spray-dried engineered particles.
Budesonide, a glucocorticoid, is known for its sticky nature – its difficulty to de-aggregate was the reason for its selection as the candidate formulation. A binary mixture of a marketed formulation of lactose and budesonide 200 μg per dose with a capsule fill weight of 25 mg was selected to test the prototype. In addition, the performance was compared with a marketed Plastiape (now Berry Global) RS01 equivalent device. The novel H&T Presspart device exhibited a high FPF compared with the marketed Berry RS01 device, confirming the engine’s efficiency (Figure 5).
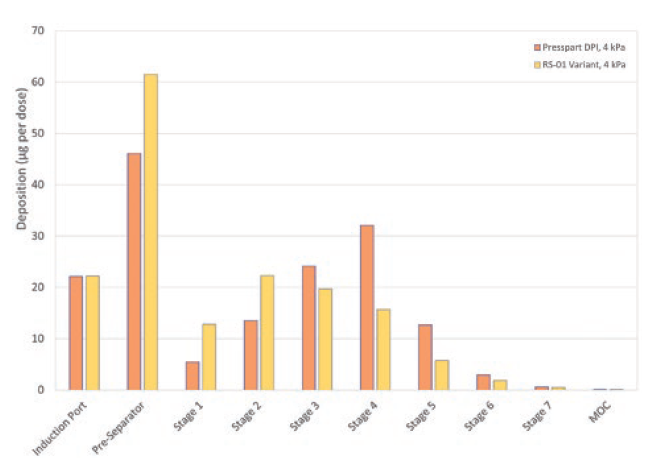
Figure 5: Comparison of NGI deposition profile of the H&T Presspart DPI and RS01 equivalent with Budesonide 200 μg powder for inhalation.
In a study in co-operation with the University of Bonn (Germany), the performance of the novel DPI was assessed by testing a spray-dried rifampicin formulation. More detailed information on that can be found in a recently published article.5 The formulation was used to benchmark the performance ofthe H&T Presspart device against devices already on the market. As shown in Table 1, the FPFs generated were highest for the H&T Presspart device when compared with the commercially available standard devices HandiHaler (Boehringer Ingelheim) and RS01 equivalent.
| Flowrate | PP-DPI | RS01 | Handihaler |
| 50 L/min | 73.4 ± 3.2 | 66.3 ± 5.5 | 47.8 ± 4.2 |
| 100 L/min | 63.5 ± 4.2 | 53.7 ± 4.2 | 46.6 ± 4.1 |
Table 1: Comparison of FPFs at different flow rates for the H&T Presspart device, RS01 equivalent and HandiHaler for the rifampicin particle-engineered formulation.
CONCLUSION
Several key factors influence the performance of the DPI. The most important ones are the patient’s inhalation technique, device handling and the device engine, in particular, the efficiency of the de-aggregation mechanism of the device. Targeting a medium resistance and a relatively flow rate independence were critical factors within the device development approach.
Various complementary techniques were used to study airflow and powder de-aggregation behaviour within a device. Combining different development approaches of fast-paced prototyping, CFD technique and laboratory data for verification can effectively develop cost-effective high-performance DPIs for the inhalation market. These techniques enabled H&T Presspart to develop a high-performance prototype independent of the formulation type tested (carrier-based as well as an engineered formulation).
REFERENCES
- Williams R, “The Download: inhaled covid vaccines, and fighting malaria”. MIT Technology Review, Sep 8, 2022.
- Geller DE, Weers J, Heuerding S, “Development of an inhaled dry-powder formulation of Tobramycin using PulmoSphere™ technology”. J Aerosol Med Pulm Drug Deliv, 2011, Vol 24, pp 175–82.
- Hoppentocht M et al, “Technological and practical challenges of dry powder inhalers and formulations”. Adv Drug Deliv Rev, 2014, Vol 75, pp 18–31.
- Dal Negro RW, “Dry powder inhaler and the right things to remember: a concept view”. Multidiscip Respir Med, 2015, Vol 10(1), p 13.
- Groß R et al, “State of the Art in Capsule-Based Dry Powder Inhalers: Deagglomeration Techniques and the Consequences for Formulation Aerosolization”. Pharmaceutics, 2022, Vol 14(6), p 1185.

