Citation: Goldinger M, Alexandersson E, “From Process to Product: Optimising Process Development Strategies for Variable Drug-Device Requirements”. ONdrugDelivery, Issue 120 (May 2021), pp 14–19.
Markus Goldinger and Erik Alexandersson discuss the benefits to process development provided by SHL’s fully in-house, vertically integrated model, and how those benefits are applied throughout the process from initial design work to final assembly of the drug delivery device and primary container.
“When the research, development and formulation of a drug can take 10 or more years, a process development strategy that can adapt to the customer’s needs is crucial to meet or even shorten timelines in combination product development, as well as mitigate project risks.”
Combination product development follows a two-pronged approach: pharma takes the lead in drug discovery and formulation development and device companies do the same for the drug delivery system. On the device side, process development (PD) takes centre stage in ensuring tangible and intangible resources are translated into self-injection devices that reach the hands of patients across the many disease areas that require them. While changes relating to the device development process and/or its intermediary products entail myriad risks, SHL Medical takes a scientific and engineering-based PD approach to safeguard the execution of all stepwise procedures undertaken in autoinjector development.
In the autoinjector industry, where buzzwords like “streamlined operations”, “end-to-end processes” and “vertically integrated services” are common and loosely used, device companies need to be challenged to substantiate such claims. For SHL Medical, three crucial elements are of prime consideration in its device development strategy:
- Formulation and mapping of processes
- Execution of these processes
- Ensuring the quality of the output of each step-by-step event leads to the carefully developed, final assembled device.
Early in the planning stages of a device project with a pharma partner, a sound PD strategy is required to ensure in-process controls are well integrated with the intended qualifiable and quantifiable outputs in the manufacturing stream. Although not commonplace, SHL believes that this should be the norm in the autoinjector industry.
CONVENTIONAL PROCESS DEVELOPMENT AT A GLANCE
In brief, PD refers to the exercise of creating a means to manufacture a specific product in a given quantity, involving the selection and sequencing of process steps from a repertoire of unit operations.1,2 Conventionally, what this means is that a device will take a highly specific development stream contingent to the project requirements – a project that requires low-volume production will undertake a distinct manufacturing process different from that which requires high-volume production. Likewise, the overarching development process for a fully bespoke device project will differ from a project that leverages the technology of a modular platform offering.
“SHL’s participation in the market launch of more than 47 combination products – designed for a wide variety of disease areas and end users – provides the company with a contextual perspective that translates to cycles of assessing, verifying and testing devices and iterating designs of its device technologies.”
Interestingly, when a device project requires not just one but a combination of these factors, the dependencies and interdependencies of upstream and downstream processes exponentially multiply and the complexities become increasingly apparent. In drug device development, the competencies of a device developer to address the requirements of the customer, primary container, branding or patient become important to the project’s success.
When the research, development and formulation of a drug can take 10 or more years, a PD strategy that can adapt to the customer’s needs is crucial to meet or even shorten timelines in combination product development, as well as mitigate project risks. For SHL Medical, the flexibility of processes is key to addressing the varying requirements of stakeholders, as well as ensuring that the manufacturing and assembly process is fully scalable.
A SCIENTIFIC AND ENGINEERING-BASED PROCESS DEVELOPMENT APPROACH
The PD strategy at SHL Medical (Figure 1) takes a scientific and engineering-based approach to ensure that the following critical considerations in combination product development are taken:
- Delineate the primary function of the device with the end-user requirements in mind during early design work
- Establish design requirements of pharma partners, establish robust and efficient processes, and deploy these into the manufacturing streams
- Ensure successful design transfer and guide the execution of the penultimate steps for the customer to undertake in the combination product development process (i.e. final assembly).
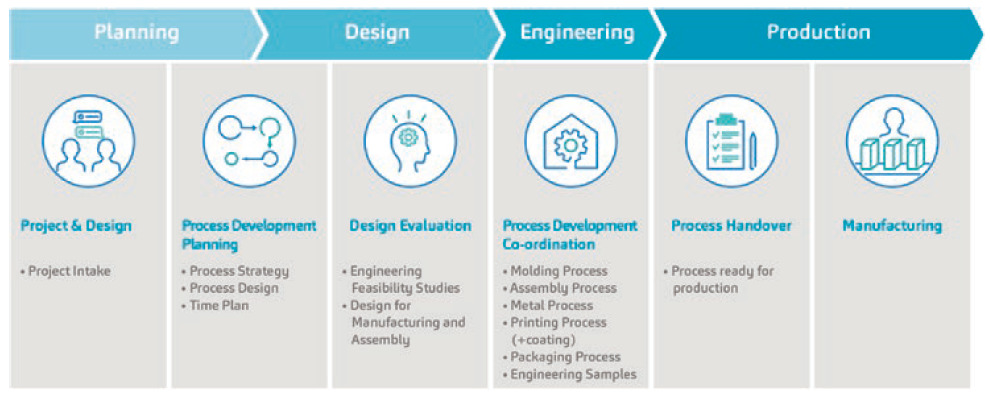
Figure 1: SHL Medical’s overarching PD streams. The validation phase and final assembly are not shown in this figure.
A key highlight of SHL Medical’s PD strategy is that it comprises both the device development aspect of a combination product project as well as the processes that directly concern its pharma partners. Specifically, this means that the PD strategy is streamlined not only for activities that constitute its autoinjector development streams, but also for the processes which would be undertaken by its customers, such as the final assembly of the autoinjector sub-assemblies with the syringe. It could be said that this PD strategy, which covers both the upstream and downstream processes of combination product development, draws from SHL Medical’s years of experience in supporting the regulatory approval and launch of a myriad of self-injection devices.
SHL’s participation in the market launch of more than 47 combination products – designed for a wide variety of disease areas and end users – provides the company with a contextual perspective that translates to cycles of assessing, verifying and testing devices and iterating designs of its device technologies. The depth and breadth of its cumulative experience have fortified SHL’s focus on a scientific and engineering-based device development approach with the end user in mind.
MANAGING DEVELOPMENT RISKS THROUGH DFMA PRINCIPLES
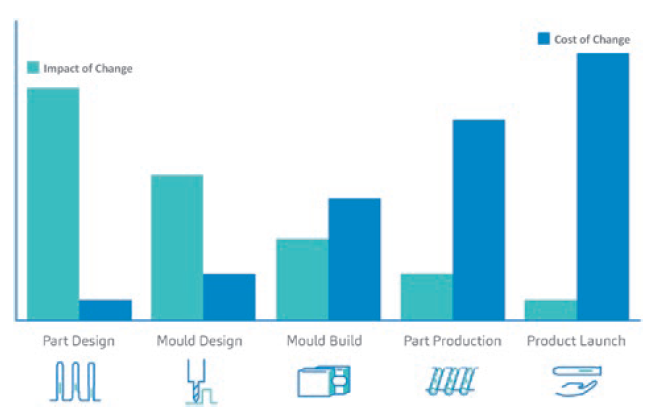
Figure 2: A generalised comparative graph representing the relationship between the impact of a change versus the cost of that change in combination product development, with a focus on the device aspect.
On the device side of combination product development, the robustness of a device technology is just one side of the story. The other side relies on an informed system of processes and procedures. An informed system means that the way the steps are undertaken downstream of the device project relies on insights from data upstream of the whole device development process. With an informed set of procedures, root causes can be identified from the upstream processes, preventing the impact of change versus the cost of change casualties.
In brief, various experts are sought to ensure that the manufacturing stream is seamless. This improves device manufacturability and mitigates project risks in relation to the impact of design changes versus the costs of running a device project. Changes brought about by data gathered from simulations in the early stages of a project will impact costs less than when a device part is already produced, or when the combination product is already launched on the market (Figure 2).3
While design for manufacturing and assembly (DFMA) concepts are not new, the PD structure at SHL Medical continuously adapts its scientific and engineering-based approach for DFMA. As a systematic solution to device projects, engineering feasibility studies, as well as DFMA evaluations, are carried out at the earliest practicable stages of product design.
An important highlight of this system is that DFMA concepts are explicitly outlined during the design proposal step in preparation for a device project’s engineering phase. This ensures that tools and process flows are well co-ordinated and integrated with mass production requirements and tuned to manufacturing capabilities, ultimately supporting the original device design requirements.
An SHL device project will undergo stringent reviews by various subject-matter experts during its design phase, well before it enters the mass manufacture stage. This would include:
- A review of the input materials, such as the plastic and metal components to be used throughout the manufacturing process
- Injection moulding and assembly simulations by computational engineers
- Moulding and assembly process reviews in accordance with historical data or prior knowledge from similar projects
- Toolset review of mould structure by tooling experts
- Review by process and automation experts of the assembly process, as well as equipment considerations for mass production.
“In contrast to a linear device development process, SHL device projects are supported through an array of in sourced capabilities, resulting in a parallel development process. These in-house capabilities ensure a vertically integrated system at SHL Medical, enabling various product development activities to have parallel, overlapping timelines.”
This framework generally serves as a “robust anchor” that guides the development – and prevents unintended issues – of an autoinjector project for a pharma partner. In accordance with the project and end-user requirements, a draft design will be made by the design engineers and a feedback loop will commence to evaluate the manufacturability of that draft design, as well as ensure that, downstream of the process, such a device can be fully assembled with the primary container carrying the drug.
Starting from the raw materials to be used, computer-aided engineering allows SHL’s subject-matter experts to understand the injection processability of the input polymers, as well as minimise warpage and shrinkage of the moulded parts. Injection moulding simulations are run to analyse and visualise how much shrinkage and warpage to expect, given the current part material, design and expected processing conditions. The results of these simulations make it possible to consider a wider range of possible solutions early in the design phase of a project, and to come up with an optimised combination of design, material and processing parameters to produce the desired part.
A keen understanding of the assembly of each moulded autoinjector part is also developed early in the device development process. Similar to injection moulding PD, computer-aided engineering is used to understand and analyse the physical force requirements of the device assembly process, such as the assembly and separation force, given the known parameters of the part material and design. This way, the functional requirements for the assembly equipment are defined and automation experts can proceed with developing the right assembly equipment for small-scale studies, as well as the mass-production phase.
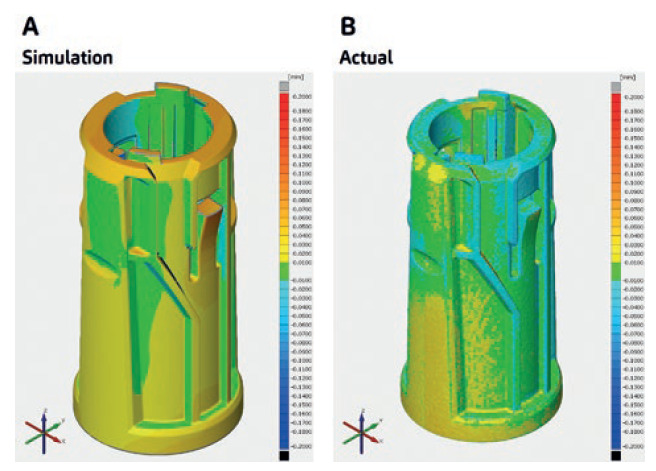
Figure 3: From simulation to actual results, a robust PD strategy ensures the desired output of the stepwise procedures along the whole device development.
When it comes to establishing the actual process, scientific injection moulding is carried out to design, as well as to produce, the right tools that conform to the design specifications, based on the initial simulation results. The first iteration of the tool is constructed and the design of experiments (DoE) is performed to test variables in the moulding process and ultimately come up with a consistent approach that would not require any modifications once the process is set. On the other hand, assembly process assessment testing is also carried out on the autoinjector prototype to support the establishment of the requirements and acceptance testing for the equipment to be used in the actual assembly process.
For SHL, consistency in results is tantamount to ensuring a robust PD strategy. This comes from understanding the whole combination product development process and the causes of issues within its streams, leading to an informed PD strategy where both simulation and actual results meet – indicating a robust process that guides the fate of the stepwise procedures along the whole device development.
Figure 3A shows warpage trend simulation data for a specific part of SHL’s modular platform device technology, and Figure 3B shows warpage data for its actual moulded part. This indicates a highly similar warpage trend for both the simulation and the actual process output, validating the robustness of checkpoints put in place along the device development stream.
FULLY IN-HOUSE CAPABILITIES
In contrast to a linear device development process, SHL device projects are supported through an array of in-sourced capabilities, resulting in a parallel development process. These in-house capabilities ensure a vertically integrated system at SHL Medical, enabling various product development activities to have parallel, overlapping timelines. For SHL, these are what truly constitute an end-to-end system of processes. This is reflected in the establishment of PD from planning through to the validation stages of a device project.
Subsequently, this vertical integration of core capabilities enables intra-organisational communications where information, whether technical or not, can be relayed directly between different functions. Using this model, communication failure is minimised. Likewise, this means that feedback, such as verifying or confirming the information received, can be provided in a timely fashion to avoid process errors. While various organisational models suffer from asynchronous communications, the vertical integration of PD capabilities equips SHL Medical with streamlined, highly responsive lines of communication across the project. This presents a considerable improvement in logistics management and helps lessen lag or idle time over stepwise work operations.
“Molly® is a device technology with proven successes and launches in the market, demonstrating its credentials as a platform device offering that has been optimised over the years. This goes hand in hand with a robust PD strategy that ensures quality in the combination and interaction of its input variables.”
A suitable analogy for describing PD within SHL is the relationship between the “spider and its web”. With most device development functions in-house (representing the spiderweb), PD experts are able to “spider” over all the steps in the autoinjector development – optimising procedures and leveraging the know-how of each specialist department (tooling, automation, verification, testing, etc) to create a robust device. In a grander scheme, this presents a multitude of advantages, including an improved time to market for customer projects, as well as enabling SHL to execute various device projects in parallel to meet their independent timelines.
INTEGRATING PROCESS DEVELOPMENT AND FINAL ASSEMBLY
As a device developer with fully in-house capabilities, SHL’s insight into the processes involved in the whole combination product development is unparalleled. Early in the autoinjector development, SHL’s final assembly experts synchronise with project teams to ensure successful design transfer and execution of the final assembly of the autoinjector with the primary container. This ensures synchronisation of deliverables during various phases of the project’s lifecycle, where SHL ensures a successful final assembly process for the pharma partner by guiding them through the process, as well as providing them with the design verification master report – technical documentation which is a suite of test methods, protocols and reports detailing the particular device.
There is depth and breadth in the informed device data that SHL analyses prior to releasing sub-assemblies to customers. This level of comprehensiveness is made possible by the expertise of SHL’s design verification and testing experts, as well as the SHL-built testing machines and in-house test method development. The composite of these also enables SHL to better understand and dissect the complexities of device design. At present, SHL the flexibility in device testing options that extend to fully automatic device testing.
It was mentioned earlier that a key element of SHL’s PD strategy is the integration of processes that have an impact not only on SHL but also on its customers. To reduce lead time for equipment design and procurement, SHL has the capacity to develop the client’s device and the required final assembly equipment in parallel. This means that in the initial stage of the device development, SHL’s in-house PD engineers collaborate closely with the equipment engineers to provide guidance on the assembly process and acceptance testing, ensuring that the final assembly process effectively aligns with the specifics of the device.4
A MODULAR PLATFORM AND A STREAMLINED PROCESS STRATEGY
It should not be forgotten that PD is always entwined with a device technology. In 2020, SHL Medical published a review of a modular platform technology; the Molly® autoinjector device.5
First launched in 2010, Molly® has since supported the development and regulatory approval of around 16 combination products covering a range of disease areas. Over the years, the Molly® technology has matured to become a modular platform that allows an appreciable level of freedom for customisation while maintaining its rotator-based mechanism. The device technology is a modular platform in the sense that both the front and rear sub-assemblies comprise five to six intricately designed parts that are configured to support flexibility in both design and manufacturing.
Molly® is a device technology with proven successes and launches in the market, demonstrating its credentials as a platform device offering that has been optimised over the years. This goes hand in hand with a robust PD strategy that ensures quality in the combination and interaction of its input variables. When it comes to autoinjectors, these input variables pertain to various layers of elements that are involved in device development, including the interacting parts that constitute a final assembled device and the network of processes involved.
With more than 20 years of experience developing drug delivery devices, SHL Medical is able to leverage a wealth of resources from within its organisation, coming in the form of platform data, subject-matter experts per variables in the PD stream and historical data that can be pulled from a multitude of past device projects that have shared various common elements. For SHL, this translates to a modular platform technology that enables the Molly® device to draw upon years’ worth of testing platform parts and sub-assemblies, as well as iterating designs, to ensure a robust device offering. This has allowed SHL to develop a platform technology that allows for customisations in the front and rear sub-assemblies of the autoinjector, supporting an autoinjector device that can address the requirements of the primary container, industrial design and usability needs of the end user (Figure 4).
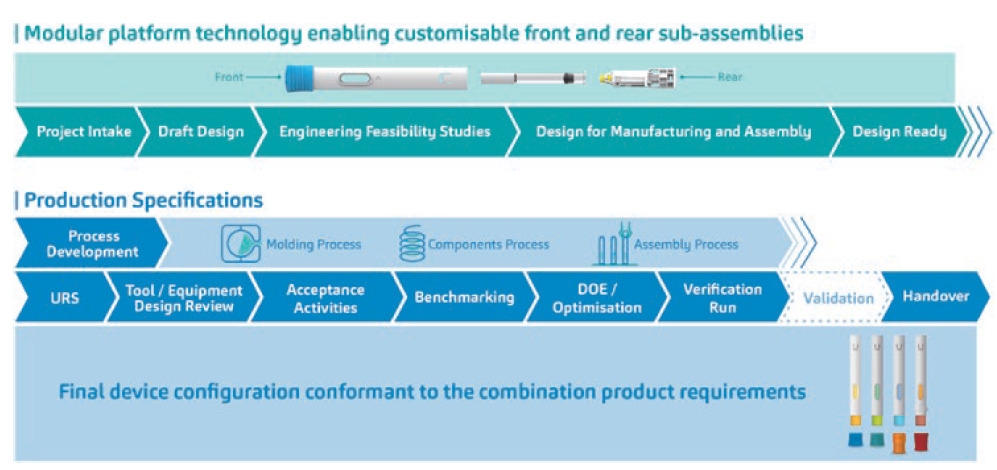
Figure 4: From process to the product, the Molly® modular platform technology, along with its robust PD strategy, allows for device configurations that conform to the highly specific requirements of any combination product. Device renderings only, not representative of final product.
CONCLUSION
SHL believes that the key to delivering self-administrable medicines in the comfort of one’s home is not only innovative device technologies, but also the sound processes that support the design, development and manufacture of combination products.
With the ever-changing needs and varying complexities in combination product development, an end-to-end process stream that includes a proven device technology enabled by a streamlined process that extends to the final assembly of the device and the primary container will be crucial to maintain a continuous and sustainable supply chain on both the drug and device development sides. SHL is disrupting the norms of the medtech space by providing solutions that cater to the end-to-end requirements of combination product development.
The authors would like to thank Kai Kiutra, Robin Wang and William Wang for their contributions to this article.
REFERENCES
- Clark JP “Chapter 6 – Process and equipment selection” in “Practical Design, Construction and Operation of Food Facilities” (Clark JP, ed). Elsevier Science, 2008, pp 85–123.
- Hejnaes KR, Ransohoff TC, “Chemistry, Manufacture and Control” in “Biopharmaceutical Processing: Development, Design and Implementation of Manufacturing Processes” (Jagschies G et al, eds). Elsevier Science, 2018, pp 1105–1136.
- Williams T et al, “The Effects of Design Changes and Delays on Project Costs”. J Oper Res Soc, 1995, 46(7), pp 809–818.
- “Final Assembly, Labelling & Packaging: An Integrated Solution for a Frustration Free Last Mile”. ONdrugDelivery Magazine, Issue 86 (May 2018), pp 6–8.
- Wild L, Fuensalida Pantig GR, “The Customisable Platform Paradox”. ONdrugDelivery, Issue 113 (Oct 2020), pp 80–85.

