Citation: “Interview: Dr Sri Mudumba, Santen & Dr Gautam N Shetty, Congruence Medical Solutions”. ONdrugDelivery Magazine, Issue 104 (Jan 2020), pp 22-24.
At the 2019 Parenteral Drug Association (PDA) Europe’s Universe of Prefilled Syringes & Injection Devices meeting in Gothenburg, Sweden, Congruence Medical Solutions and Santen Pharmaceutical were presented with the Drug Delivery Innovation Award for their partnership to develop the Microliter Dosing Syringe (MDS) device platform. Award criteria included – being an innovation, solving an unmet need in the market, global market relevance and combining different technical expertise in developing a technical solution. Sri Mudumba, PhD, of Santen, and Gautam Shetty, PhD, of Congruence, received the award on behalf of their respective organisations.
Dr Mudumba is Vice-President of Therapeutic Modality Innovation at Santen – a global company focused solely on ophthalmology and committed to serving serious unmet needs by developing innovative solutions that protect and restore vision. He has been working in ophthalmology research and development for 15 years. Dr Shetty is the founder of Congruence Medical Solutions. Prior to Congruence, he was General Manager of a novel drug delivery systems business unit at Unilife. He pioneered development of ophthalmic drug delivery devices and targeted organ delivery systems.
In this interview, Drs Mudumba and Shetty share insights from their experience of working together in developing the MDS for ophthalmic injections.
Q Why did you identify the need for your ophthalmic drug product in a prefilled format?
SM Our product profile required an injection as low as 20 μL. I had investigated various options for prefilled syringe manufacturers and fill-finish contract manufacturing organisations (CMOs) and couldn’t find anything that could deliver precisely 20 μL in prefillable format. We approached Congruence with this challenge and they came up with a solution. A prefilled syringe offers a lower fill volume (reduced drug product waste and increased batch yield ultimately offering lower cost of goods sold) than a vial. In addition to the product yield, from a customer-centric approach, a prefilled syringe is preferred as it involves less manipulation before injecting.

Figure 1: Gautam Shetty (right) and Sri Mudumba (centre) receiving the 2019 Drug Delivery Innovation Award from Thomas Schoenknecht (left), Chairman of the PDA Conference and member of the Awards Committee. (Image Courtesy PDA.)
GS It has been fascinating to observe the ophthalmic injection space since Lucentis (ranibizumab) was launched in June 2006 by Genentech (now part of Roche) – an incredible pharma innovation that transformed what was a suboptimal surgical intervention to treat blinding eye disorders into a successful pharma intervention. Since then, a growing number of pharma companies have innovated and continue to develop novel treatments that need injections in the eye – Santen being one of them. The value proposition for prefilled syringes as applied in subcutaneous and intramuscular injection – streamlining injection procedure, reducing costs, driving customer preference, product differentiation – holds true in ophthalmology as well.
Q What are key requirements for an ophthalmic injection system? Are there unmet needs outside of ophthalmology?
SM It is critical that sterility is maintained throughout the eye injection procedure (reducing the number of steps in preparation of injection) to prevent any potential for infectious endophthalmitis. It is also important to minimise the burden on the physician for accurate dosing. Another factor that is becoming increasingly important is to minimise injection of non-drug impurities such as silicone oil into the eye.
GS There are a number of regulatory and compendial requirements unique to an ophthalmic drug delivery system. The commercialisation path of least resistance for a prefilled option requires incorporating standard container closure components into an ophthalmic injection system. Ophthalmic drugs are delivered in microlitre volumes, typically 50 μL or lower. Delivering a microlitre dose accurately and precisely using a syringe that typically delivers millilitre volumes becomes a key requirement. Also, considering that several drugs are still in clinical development, a microlitre injection system should be able to draw from a drug vial and then deliver an accurate, precise microlitre dose. In addition to the applications in ophthalmology, there are several emerging applications in oncology, neurology, dermatology and localised delivery that require delivery of microlitre-size volumes.
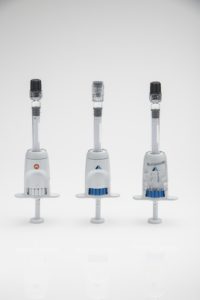
Figure 2: The Microliter Dosing Syringe (MDS) incorporates standard prefillable syringes, shown here with various 1 mL long prefillable syringes.
Q Why and how did you decide to manage development of the MDS through an external partnership?
SM Santen’s core expertise is not in injectable drug delivery devices and associated primary packaging. We constantly evaluate external partnerships that offer new technologies that will help our products in tiers. Microlitre dosing serves as an enabling technology when the existing commercially available syringes do not meet our criteria for low-volume accurate dosing. When trying to bring a best-in-class therapeutic onto the market, the delivery plays a big role. The MDS format (Figure 2) can be a key differentiator with a competitive advantage. In addition, as it takes a long time to ultimately commercialise a drug product, a device as a lifecycle-management tool becomes very critical for us. MDS can help us manage the lifecycle of the drug product as well. Finally, our core expertise lies in drug product development and not device development, so we decided to look for external partners who can help us build a prefilled syringe-based platform.
GS There has always been a decision to make when an off-the- shelf delivery system is unavailable. Make or buy? Reasons for either option have been fairly well understood and broadly discussed in the industry. In this case, our background and specialised competence to develop a novel technology with the performance necessary for accurate, precise delivery within the constraints of standard pharma fill-finish infrastructure positioned us as the partner of choice.
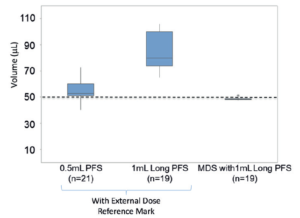
Figure 3: Ophthalmologists attempted to deliver a 50 μL dose using the MDS and conventional prefilled syringes.
Q What were key factors in making this partnership successful?
SM It takes a village.
GS In addition to development sponsor and device developer, there was buy-in from primary container component suppliers, CMOs and filling equipment manufacturers. It was a situation where there was a convergence and alignment of interests. Several syringe suppliers, who may have been left out of the microlitre dosing space, looked at the MDS as an enabling technology. A syringe that typically delivers millilitre doses can now deliver accurately and precisely a microlitre dose. Figure 3 shows results from a study in which ophthalmologists attempted to deliver a 50 μL dose using the MDS and conventional prefilled syringes, and which demonstrates how effective the MDS is in improving dosing accuracy. In addition, the Santen team delegated a lot of decision making to Congruence, which was very important to move the device development fast. The Santen team’s integrated approach included involvement in all aspects of drug fill-finish tasks even if it did not involve a device component. This ensured that decisions in the drug fill-finish process did not blindside the device development team.
Q What’s next for the MDS?
SM This platform could help us get products into human trials with very minimal dosing as there are not many options for filling at the time of use for low-volume filling. It can also reduce cost of expensive modalities with low fill volumes, and improve shelf-life stability where vial headspace is not desired.
GS The MDS platform can be made available as a prefilled syringe format or so that drug can be filled from a vial, and its injection dose volume can be preset by the manufacturer, or set by the user. This gives four different combinations of configurations (Figure 4). The MDS in its non-prefilled configuration enables a prefillable syringe to be paired with a vial-filled drug. We have initiated submission of the MDS for US FDA 510k premarket clearance. expect in 2020 the MDS will be available for use in drug clinical trials or drug commercial sales.
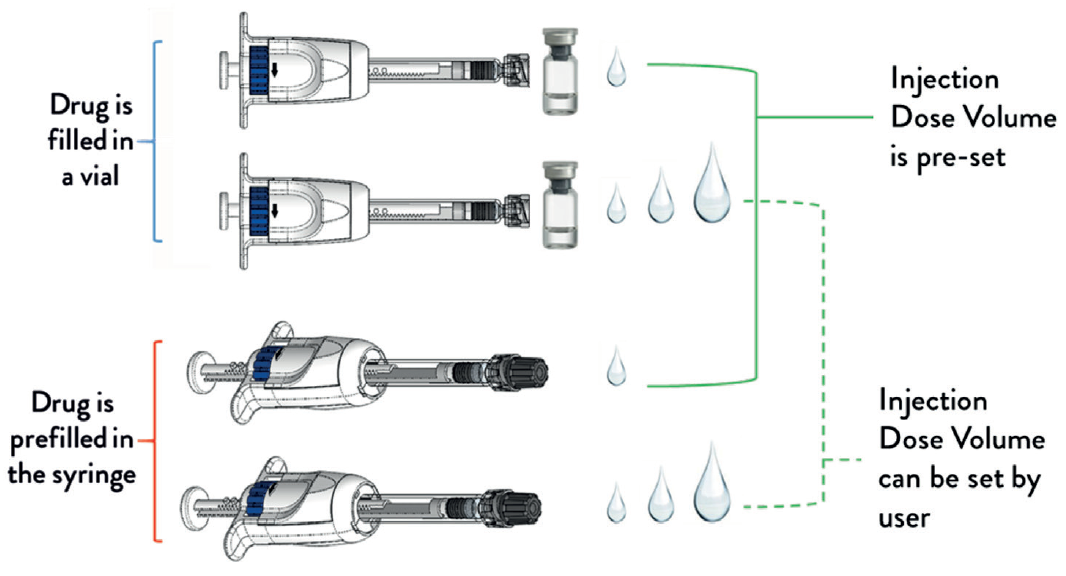
Figure 4: The MDS platform can be made available in four different configurations.
Previous article
OPTIMISING OPHTHALMIC DRUG DELIVERY FOR THERAPEUTIC EFFECTIVENESSNext article
VIDEO: Credence Connect from Credence MedSystems
