To Issue 151
Citation: Bohannan R, “Injecting Innovative Solutions into Connected Delivery Devices”. ONdrugDelivery, Issue 151 (Sep 2023), pp 66–68.
Roger Bohannan discusses the capabilities and design challenges of connected drug delivery devices.
“The main market-driving factors are the prevalence of chronic illnesses, technology advancements and an increased desire for in home care.”
Traditional analogue medical devices, such as autoinjectors, are now being designed to leverage the capabilities of the Internet of Things (IoT). Connected medical devices provide enhanced monitoring, data collection, connectivity and, more importantly, patient safety. Patient acceptance to mission-critical connected pharmaceutical delivery devices is dependent on innovative designs that offer ease of use, accuracy of dosage delivery and affordability. Autoinjectors, inhalers and wearable infusion device designs require component technologies that ensure proper and safe performance. In addition, designs must go through rigorous testing to meet global and regional safety standards and regulations.
MARKET FORECAST
There are an estimated 1.3 billion medical injectors used today in hospitals, medical centres and homes around the world. According to Grand View Research, the global injectors market size was valued at US$6.66 billion (£5.24 billion) in 2021. The market is expected to expand at a compound annual growth rate of 15.29% from 2022 to 2030.
The main market-driving factors are the prevalence of chronic illnesses, technology advancements and an increased desire for in-home care:
- Chronic illnesses: an ageing population and the rising prevalence of chronic ailments such as diabetes, cardiovascular disease, obesity, cancer, psoriasis, arthritis and migraines. Currently, the highest volumes of injectable medicine use are for Type 1 diabetes, psoriasis, arthritis and migraines.
- Technology innovations: technological advancements, both in terms of the delivery system and the development of new drugs. Many drugs require special delivery devices to be administered efficiently to patients. The rapidly expanding biopharmaceutical industry is driving the development of innovative drugs, which in turn encourages the growth of the global injector market.
- In-home care: rising demand for home-based treatments as they require less hospitalisation, minimal expertise and lower medical costs.
“Moving to IoT connected injectable devices holds great promise in revolutionising healthcare by providing more comprehensive and patient-centric solutions.”
EMERGING MEDICAL INJECTOR CAPABILITIES
Analogue medical devices refer to traditional, non-electronic devices that are typically used for therapeutic or diagnostic purposes. These devices have an enormous amount of engineering and science designed into the delivery system. Today, designers are adding even greater device functionality. This includes increasing the complexity of the device, while not impacting the mechanical performance of the delivery system. However, analogue medical injectables are limited in their ability to provide real-time data and patient insights. As a result, doctors are dependent on patient insights, record keeping and results alone. IoT connected devices incorporate digital technologies, sensors and wireless connectivity to collect data and provide real-time monitoring (Figure 1).
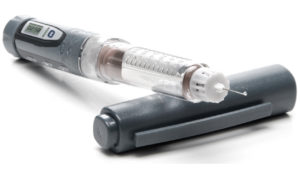
Figure 1: Example of a connected medical injectable device.
IoT connectivity facilitates data exchange with other devices or cloud-based platforms. Moving to IoT connected injectable devices holds great promise in revolutionising healthcare by providing more comprehensive and patient-centric solutions. A connected autoinjector, inhaler or wearable infusion device that has US FDA approval, a CE mark or Chinese NMPA (National Medical Products Administration, previously the CFDA) certification allows for the data from these devices to be actionable for medical professionals. IoT connectivity offers the following advantages compared with traditional analogue devices:
- Enhanced monitoring and data collection: collects and transmits data on various parameters, such as medication delivery timing, dosage and patient responses. This data can be invaluable for healthcare professionals in making informed decisions and adjusting treatment plans.
- Personalised healthcare: allows for personalised healthcare solutions. By continuously monitoring patients and collecting data, these devices can provide tailored treatment plans based on individual needs and responses. This personalisation can lead to better patient outcomes and improved quality of life.
- Remote patient monitoring (RPM): enables remote patient monitoring, where healthcare providers can track patients’ health status without the need for frequent in-person visits. RPM can be especially beneficial for patients with chronic conditions, as it allows for early detection of potential issues and timely interventions.
- Data integration and analysis: seamlessly integrates with other healthcare systems and platforms, such as electronic health records or telemedicine platforms. This integration streamlines data analysis, enables data-driven insights and facilitates better communication between patients and healthcare providers.
- Medication adherence and compliance: monitors medication adherence and alerts patients or healthcare providers if doses are missed. This feature can significantly improve patient compliance with prescribed treatments.
- Improved safety features: offers safety features, such as dose-limiting and tamper-detection mechanisms, to ensure the device is used correctly and prevent misuse.
- User engagement and experience: features interactive user interfaces that enhance the overall user experience of patients. This can lead to increased patient engagement and better treatment adherence.
- Data security and privacy: addressing concerns about data security and patient privacy, original equipment manufacturers (OEMs) are deploying the most advanced remedies available today and continue to evolve as the threat evolves. Today, they are implementing robust data-encryption and authentication mechanisms to safeguard sensitive health information.
“Users desire sleek and compact medical injector devices with enhanced functionality.”
DESIGN CHALLENGES
As IoT technology has become more accessible and affordable, the cost of manufacturing and deploying connected medical injectables has decreased significantly. This scalability enables wider adoption of such devices, making advanced healthcare solutions available to more patients. Users desire sleek and compact medical injector devices with enhanced functionality. IoT connected devices must also have a simplified interface with pleasing haptics that confirm actuation.
Ensuring precise dosage accuracy, confirmation of dosage delivery and recording of patient data in a small package can be a significant challenge. In addition to small size, weight and power requirements play a role in designs. Patients want lightweight devices with a long battery life. Designs must also feature rugged and reliable construction that meets environmental performance testing, such as drop tests and temperature cycling. Combining all these requirements can lead to a complex design cycle. Working with experienced partners who navigate these challenges daily helps speed up the design cycle.
Because of the mission-critical nature of connected medical injectable devices, designs often require the customisation of miniature IP67-rated components. IP67-rated components are sealed for protection against solid objects such as dust and sand, as well as liquids such as water or sweat.
From a patient protection standpoint, data encryption and security are vital. Implementing security features, such as encryption modules and secure authentication mechanisms, protects sensitive health data transmitted over the internet.
“Navigating the complex nature of connected medical injectable design requirements with global and region/nation-specific design regulations can be challenging.”
Autoinjectors, inhalers and wearable infusion devices must also meet various government and safety testing standards to ensure their safety, effectiveness and compliance with regulations. The specific standards applicable to medical devices depend on factors such as the device’s intended use, classification and the geographical region in which it will be marketed. Here are some commonly referenced standards and regulations for medical injectable devices:
- FDA: in the United States, the FDA sets forth regulations for premarket approval, quality system regulations, labelling, adverse event reporting and post-market surveillance. The specific regulatory pathway and requirements depend on the device’s classification (Class I, II or III) and the associated level of risk.
- Medical Device Directive/Regulation: in the EU, medical injector devices must conform to the Medical Device Regulation (MDR) or the previous Medical Device Directive (MDD). These regulations outline requirements for conformity assessment, clinical evaluation, post-market surveillance and labelling.
- ISO 11608-1: an international standard that specifies the requirements and test methods for needle-based injection systems intended for medical use. These systems are designed to deliver drugs or medicinal products to patients via subcutaneous, intramuscular or intradermal injections. The standard outlines the essential characteristics of these injection systems to ensure their safety, reliability and performance in medical applications.
- ISO 11608-4:2006: an international standard that specifies requirements and test methods for electromechanically driven injectors intended to be used with needles and with replaceable or non-replaceable cartridges. The injector may be for single use or multiple use.
- IEC 60601: an international standard that specifies the essential safety and performance requirements for medical electrical equipment and medical electrical systems. The standard is designed to ensure the safety of patients, medical personnel and others in the vicinity of the equipment. It covers a wide range of medical devices and technologies, such as diagnostic equipment, monitoring devices and surgical instruments, to ensure they meet the necessary safety and performance criteria for medical use.
- IEC 60529: an international standard that outlines the degrees of protection provided by enclosures of electrical equipment against the intrusion of foreign objects (such as dust and water) and the access of human body parts to hazardous parts inside the enclosure. The standard is commonly referred to as the ingress protection code (IP code) and is followed by a two-digit number (e.g. IP65) to indicate the specific level of protection provided by the enclosure. This standard is widely used in various industries to define the environmental protection capabilities of equipment and to ensure their safe and proper operation in different conditions.
- IEC 60068: a collection of methods for environmental testing of electronic equipment, components and electromechanical products to assess their ability to perform and survive under conditions such as transportation, storage, operational environments and extreme cold and heat.
STREAMLINE SOLUTIONS
Navigating the complex nature of connected medical injectable design requirements with global and region/nation-specific design regulations can be challenging. Manufacturers must stay informed about the latest requirements and consult with regulatory experts to ensure compliance with the most up-to-date standards in the relevant jurisdictions.
OEMs that partner with component manufacturing companies that have dedicated medical engineering and product development teams can help simplify the design cycle. Dedicated medical teams are accustomed to handling design challenges ranging from miniaturisation requirements and tolerance stack-ups to environmental testing performance and confirmation of data accuracy under extreme testing environments. They collaborate with OEMs throughout the entire journey of product development. From product inception to prototyping and IEC and ISO testing standards, and all the way through to product launch, dedicated medical component partners keep development costs down and get autoinjectors, inhalers and wearable infusion devices to market faster with a better solution.

