To Issue 152
Citation: “Interview with Claire Raynal-Olive and Pierre-Yves Favennec, Aptar Pharma”. ONdrugDelivery, Issue 152 (Oct 2023), pp 130–133.
Claire Raynal-Olive and Pierre-Yves Favennec discuss the strategy and objectives of Aptar Pharma’s expansion programme of its global rubber component manufacturing, implementing advanced technology, increased capacity and local-for-local manufacturing capability.
“We’ve focused this expansion on three key pillars – expanding our capacity, improving our capabilities and increasing our proximity to our partners.”
Q Can you start by giving us a brief overview of Aptar Pharma’s place in the pharma and drug delivery sectors, particularly where it comes to injectable drug delivery?
CRO Absolutely, Aptar Pharma is a major manufacturer of drug delivery systems and components. We provide a wide variety of products across multiple delivery routes, including nasal, respiratory, eye-care, dermal and injectables. With regard to injectables specifically, we offer a range of best-inclass components for vial, prefilled syringe, autoinjector and cartridge applications, such as our state-of-the-art PreumiumCoat® ETFE-coated elastomeric components and our comprehensive catalogue of rigid needle shield designs and formulations. We’re active across a number of applications throughout the sector and support the development and commercialisation of biologics, vaccines, small molecules, anti-thrombotics and veterinary medicines.
To give you an idea of our scale, Aptar Pharma components are used in the delivery of more than 13 million injections in over 70 countries every day. So, as you can see, we’re a truly global player. Coupled with our full-service offering and comprehensive range of components, Aptar Pharma’s worldwide reach makes us one of the go-to partners for anyone looking to market and manufacture injectable medicines.
Q Given Aptar Pharma’s position in the market, how are you planning to build on this success and improve your offering for current and future partners?
CRO Aptar Pharma is more than just a component producer, we’re a full-service provider able to help our partners achieve success at every stage of the product development journey. To ensure that we can offer the best possible service to our partners, Aptar Pharma’s injectables division is making excellent progress through a massive programme of investment to expand our capabilities worldwide, with several elements already delivered. For a major investment such as this, it’s important to ground the programme with a clear guiding principle, based on a clear understanding of current market needs and how to address them. Therefore, we’ve focused this expansion on three key pillars – expanding our capacity, improving our capabilities and working more closely with our partners.
We’ve set ourselves ambitious goals and are already transforming expectations of what an injectables partner can be. The first part of this, the first pillar, is expanding our production capacity to meet the increasing market demand we’re seeing in the injectables sector. This is critical for us, as the greater capacity will enable us to guarantee long-term supply security for our partners. At the sametime, we’re expanding our capabilities in accordance with our second pillar by investing in state-of-the-art manufacturing technology that will contribute to improved product quality and reliability.
A fundamental principle of our current expansion is to regionalise our operations so that we can serve the needs of our partners more locally, as part of the third pillar. We’re constructing an additional factory at our Granville site in France, expanding our capabilities at our Congers site in New York (US) and our acquisition of Hengyu in Weihai, China, give us substantial manufacturing capability there. With factories in France, the US and China, as well as our global network of sales offices, we can be even more responsive and efficient in meeting the needs of our partners around the world (Figure 1).

Figure 1: Aptar Pharma is making excellent progress with its expansion and investment programme, including new and expanded facilities.
Q Which key capabilities is Aptar Pharma aiming to improve on with this expansion?

Figure 2: Aptar Pharma is implementing ISO 5 and 7 cleanrooms in its facilities to minimise the risk of particulate contamination.
PYF Along with increasing our manufacturing footprint, we’re investing in our production methods to enhance quality and reliability. This evolution of our process includes implementing state-of-the-art robotisation in our facilities to increase the reproducibility of our processes and reduce the risk of contamination from human interaction with the product on our production lines. In conjunction with this, we’re adding ISO 5 and 7 cleanrooms to minimise the risk of particulate contamination throughout the process (Figure 2). We are also expanding our automated vision control with new machines on some of our finishing lines, which will contribute to a lower incidence of defects in our products and therefore rejects on customers’ filling lines. Additionally, we’re moving toward more digitalisation in our factories, with the aim of improving traceability and ensuring consistent quality control.
Our investments focus on injectables components that address key market needs. This includes our PremiumCoat® products, PremiumFill®manufacturing process, ready-to-use (RTU) gamma-sterilised components and rigid needle shields for prefilled syringes and autoinjectors.
Whilst our products are the key focus of the current expansion, it is important to note that Aptar Pharma is a full-service provider that supports partners at every stage of the drug development journey, from primary packaging selection and formulation development, through clinical trials, regulatory filing through to market launch, and including patient onboarding solutions and digital therapeutics.
“As a full-service provider, Aptar Pharma is ideally positioned to partner with from step one, assisting with selecting the right packaging for our partners’ drug products from early on, avoiding incredibly costly delays, or even product reworks, down the line.”
Q With a key aim of Aptar Pharma’s investment being to improve the company’s ability to meet its partners’ needs, can you elaborate on what trends you see currently shaping the injectables market?
CRO It’s of great importance to us to keep abreast of market trends so that we can anticipate the needs of our partners and offer solutions adapted to those needs. Aptar Pharma has identified three key trends that are having a major influence on today’s market – first, strong market growth; second, continued growth in chronic disease and biologic treatments; and, third, rising R&D costs associated with bringing a drug to market.
On that first point, despite recent turbulence, the injectables sector is continuing to see strong market growth across all applications, especially in chronic therapies and antidiabetics. Injection is now the number one route of administration and its market value expected to increase from US$606 billion (£477 billion) in 2020 to $1,600 billion by 2027. This means that our partners require a future-proofed supply of components with embedded surge capacity to absorb fluctuations in the market. Recent years have highlighted the necessity for providers to have the capacity to respond to global events, such as the covid-19 pandemic. Aptar Pharma’s investment programme will increase our production capacity by 40% to meet the long-term needs of our partners.
Q You mentioned that one of the three key market trends is the rise in biologic treatments – how significant is this factor and how is Aptar Pharma responding to it?
CRO There’s major growth in the number of biologic treatments in development and making their way to market. Over the past few years, while there hasn’t been a significant increase in the total number of drug approvals, the proportion of biologics receiving approval from regulatory authorities has increased from as low as 7% in 2000 to 50% in 2022. This incredible surge in biologics is due to their ability to meet unmet needs in the chronic disease space, which itself is growing as a result of ageing populations, as well as their applicability to immune disorders and oncology. On top of this, there’s a lot of interest in biologics due to their lower incidence of side-effects compared with traditional small-molecule therapeutics, which makes the additional difficulty in manufacturing them worthwhile.
Indeed, biologics, which are mainly represented by recombinant proteins, monoclonal antibodies or even nucleic acids, are more sensitive than traditional drugs and have much more elaborate structures. In most cases, the preferred delivery route for biologics is injection and solutions like PremiumCoat® are specially designed to help our partners tackle the challenges of manufacturing these complex drugs by protecting them from extractables and leachables, ensuring their long-term preservation once packaged.
Innovation in injectable drug delivery is also allowing for another trend we’re seeing in the sector, that being the focus on moving care from the hospital to the home. Modern prefilled syringes and autoinjectors are enabling patients to take control of their healthcare and administer their own medicines in their own homes, which is both more convenient and more sustainable; however, with patients administering drugs rather than healthcare professionals, it becomes all the more imperative that injection devices are convenient and user-friendly, driving the popularity of easy-to-use devices like autoinjectors. Naturally, demand for key components such as Aptar Pharma’s PremiumCoat® stoppers and plungers will rise in concurrence with increased autoinjector use, so we’re both expanding our capacity and upgrading our equipment to ensure reliable supply and optimum quality.
Q You mentioned that the costs associated with R&D are increasing, how can Aptar Pharma assist partners who may be struggling with this?
CRO In 2010, the average cost of new drug development was $1.8 billion, by 2015 it was $2.6 billion, and it’s only getting more expensive. Biologics may be a significant factor here, as they cost more both to develop and to manufacture. The ever increasing regulatory requirements are also a factor that contribute to making new drugs more expensive to develop. The most recent example is the new EMA GMP Annex 1 revision that puts further pressure on manufacturers who now need to demonstrate comprehensive contamination control strategies that cover their whole supply chain, including primary packaging (see this article from Aptar Pharma, for more on this subject).
With these rising costs, derisking projects and regulatory compliance have become critical concerns for drug developers, who want to reach market as early as possible. One of the ways to achieve this is to enter partnerships with expert suppliers as early as possible in the product development journey. To derisk the development of sensitive drug products, Aptar Pharma offers a range of solutions, such as PremiumCoat®, and the newly updated PremiumFill® solution, which can dramatically help customers meet the requirements of the Annex 1 revision.
As a full-service provider, Aptar Pharma is ideally positioned to partner with from step one, assisting with selecting the right packaging for our partners’ drug products from early on, avoiding incredibly costly delays, or even product reworks, down the line. To add to that, we offer a wide range of services in-house to help shepherd a project through its initial stages and on to regulatory submission and market release, accelerating, lowering risk and increasing efficiency all the way through.
Q It’s clear that increasing capabilities and capacity are at the core of meeting current market needs. Can you give any further insights on Aptar Pharma’s expansion programme?
PYF We’re expanding in a big way. Over the last three years, we’ve already implemented a significant portion of our 40% capacity increase programme. To achieve this, we’ve extended our Granville factory, focusing on creating extra capacity for PremiumCoat® and PremiumFill® solutions. We also expanded our site in Le Vaudreuil (France), with a focus on rigid needle shield assembly. Furthermore, our new second factory in Granville and the expansion of our Congers facilities are expected to come online in the first quarter of 2024, which will be a huge boost for us. The Congers expansion, focused on PremiumCoat®, is bringing manufacturing to the US and providing our US-based partners with a local production centre.
We’ve markedly ramped up our production capacity in a relatively short time. But we haven’t simply added additional factory space, we’ve focused on operational excellence, which has included improving our capacity for high-value products for the industry, implementing sophisticated technologies to improve the quality of our products and we’ve thought strategically about how to use our expansion programme to expand geographically to bring us closer to our partners.
Q You said you’ve put a keen focus on both improving operational excellence and increasing production capacity, can you explain how Aptar Pharma achieves this?
PYF There are five key themes we’ve kept in mind for ensuring that we can offer best-in-class services to our partners. First and foremost, we’ve put a major emphasis on the safety and wellbeing of our employees – this is a fundamental component of Aptar Pharma’s core values. This means that we’ve built risk reduction, safety and ergonomics into our new facilities by design. As a business, Aptar Pharma puts sustainability at the heart of our operations, and taking care of our teams is part of that.
The second theme we’ve focused on is quality. We want to deliver the highest possible quality for every component. A key aspect of this theme is implementing uninterrupted cascading cleanrooms in our facilities, minimising the risk of contamination at all stages of production. Another aspect is our implementation of full traceability across our production lines, utilising process and machine connectivity for data collection. We have also put innovative in-process vision control in place to further reduce defects.
Together, the next two themes – digitalisation and robotisation – are part of our commitment to modernisation and bringing in the latest technologies to augment our production capabilities. We’ve implemented robotisation to automate and semi-automate aspects of production at every stage, including mixing, moulding and finishing, reducing the need for manual labour in our manufacturing processes (Figure 3). The implementation has also given us a chance to review facility layouts and optimise them. Alongside this, digitalisation has enabled us to enhance the ways we monitor the production process and support Industry 4.0 readiness in our facilities, taking further steps towards full connectivity.
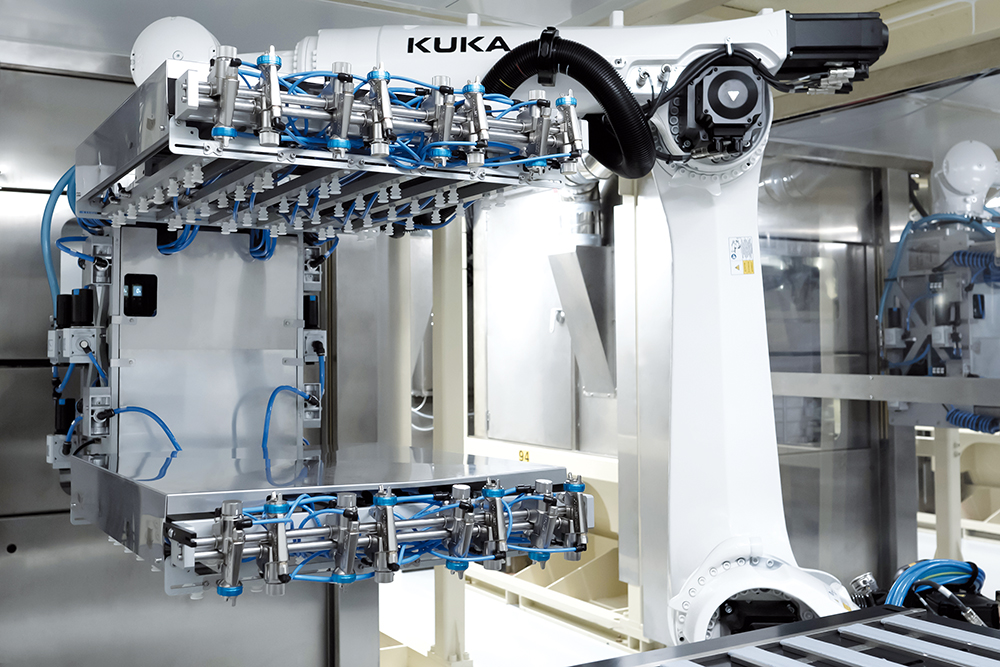
Figure 3: Aptar Pharma is using robotisation to automate its production processes.
Lastly, learning the lessons of recent years, our fifth key theme is ensuring that we’re fully prepared for surge demand. We understand that when an unexpected spike in demand occurs, we need to be able to guarantee reliable supply. To meet this need, we’ve implemented modular layouts for our moulding and finishing workshops to ensure that we can efficiently adapt our production mix to the surge demand. Furthermore, our support teams are trained to work cross-functionally and ready to respond as and when the need arises.
In summary, we’ve taken a strategic approach to this expansion, with clear objectives in mind. We’re transforming the expectation of what an injectables partner can be, and we’re eager to show what we’ve achieved; Aptar Pharma can provide its best-in-class injection device components with even greater quality and reliability than ever before.
To learn more about Aptar Pharma’s injectables offering, visit: www.aptar.com/pharmaceutical/delivery-routes/injectables.

