To Issue 143
Citation: Takeuchi K, Whelton R, “Optimising Microlitre Dosing: Case Study with Intravitreal Injection”. ONdrugDelivery, Issue 143 (Mar 2023), pp 44–49.
Yuki Takeuchi and Richard Whelton discuss the challenges of delivering intravitreal injections and how the combination of Terumo’s PLAJEX™ prefillable syringes with Congruence’s Microlitre Dosing Syringe system is able to address them.
RISING IMPORTANCE OF INTRAVITREAL INJECTION PFS
Intravitreal injections are used to deliver drugs into the vitreous humour of patients’ eyes. At present, this route of administration is typically used to deliver anti-vascular endothelial growth factor (anti-VEGF) drugs to treat ophthalmic disorders such as age-related macular degeneration, diabetic macular oedema and diabetic retinopathy.
“The number of intravitreal injections is expected to continue growing beyond the current estimated 20 million injections per year.”
The number of intravitreal injections is expected to continue growing beyond the current estimated 20 million injections per year,1 driven by an increasing number of diabetic patients, a rapidly ageing global population, the entry of biosimilars into the ophthalmic space and the emergence of new treatments for additional conditions, such as geographic atrophy.
Prefilled syringes (PFSs) are widely used for intravitreal injections because of their advantages over vials, including reduced injection time, possible reduced risk of endophthalmitis and a reduction in intraocular silicone oil droplets.2
KEY CONSIDERATIONS FOR SELECTING A DELIVERY SYSTEM FOR INTRAVITREAL INJECTION
Since intravitreal injection is a direct injection into a privileged organ, it faces different requirements and challenges to those of traditional parenteral injection, such as subcutaneous or intramuscular injection. Therefore, thoughtful considerations are needed to select an appropriate drug delivery system to ensure safe and effective treatment. Below is a summary of key considerations for selecting a drug delivery system for intravitreal injection.
Silicone Oil Droplets
Traditionally, silicone oil has been used as a lubricant for syringes to facilitate smooth movement of the plunger stopper within the syringe barrel. However, in the case of ophthalmic drugs, it has been shown that silicone oil can be deposited in the eye’s vitreous humour and accumulate after repeated injections.3
Silicone oil droplets that remain in the eye and are visible to the patient are commonly referred to as “floaters” and avoiding them is one of the key considerations for intravitreal injection treatments. Several studies now recommend the use of silicone oil-free syringes for intravitreal injections to address this.4
Sub-Visible Particles
Ophthalmic drugs are required to follow stricter requirements compared with other parenteral drugs. For example, USP<789> “Particulate Matter in Ophthalmic Solutions” requires ophthalmic drug products to have no more than 50 particles sized ≥10μm per mL, and no more than five particles sized ≥25μm per mL. Silicone oil is also known to be detected as a particle in this context and to have the potential to induce protein aggregation, which further increases particle counts in drug products.5
Accurate Dosing
For most marketed intravitreal injection drug products, the typical administration volume is 50 μL but actual injected volume can vary significantly.6 In one study, presented at the American Academy of Ophthalmology meeting in Chicago, IL, US in October 2018, the mean deliverable dose using a traditional delivery system was shown to be 53.75 mg compared with an expected mass of 50 mg, with a range of 24.6–65.5 mg.7 This can be a significant issue when the therapeutic window is extremely narrow, such as with potent drugs or pre-term neonates diagnosed with retinopathy of prematurity. Additionally, accuracy and precision of the delivered dose are critical to the fidelity of clinical data used to measure drug effectiveness, such as in dose-ranging studies.
There are several factors at play when attempting to achieve accurate dosing, such as tight tolerances of the internal diameter of the syringe. For syringes with an external dose mark, the factors impacting dose accuracy also include precise dose mark printing and user-related factors, such as ensuring that the correct part of the plunger is aligned with the marking on the barrel and that the plunger ends in the correct position after injection.
“An option for addressing the challenges in intravitreal injection is to combine a silicone oil-free primary container designed for low-dose applications, such as Terumo’s PLAJEX™ ready-to-fill polymer syringe, and a mechanical, precision-dosing module, such as Congruence’s MDS.”
Terminal Sterilisation
In order to comply with regulatory requirements and mitigate the risk of infectious endophthalmitis, drug delivery systems for intravitreal injection are required to be processed with terminal (post-filling) sterilisation. Therefore, it is critical to select a delivery system that is compatible with a suitable terminal sterilisation method for the finished drug product.
Minimising Drug Fill Volume and Wastage
Most injectable drug delivery systems involve some drug wastage, due to overfill, dead space and vial transfer. However, due to the small volumes involved in ocular delivery, the proportion of waste is much greater. For example, a 50 μL dose of Lucentis (ranibizumab, Genentech) requires a fill volume of 165 μL in a PFS format, which equates to 230% wasted dose.3 The percentage waste is even greater when vials are used. Beyond the implication for cost, minimising drug wastage aligns with sustainability objectives.
Usability
The number of injections retina specialists need to administer is continuously increasing. This places a focus on solutions that enhance usability, streamline use and improve workflow efficiency, enabling increased patient throughput.
Use Steps
Fewer device use steps enhances usability and may also confer other benefits, such as lowering the risk of exogenous endophthalmitis arising from infection in the eye. Endophthalmitis is considered one of the most serious adverse events related to intravitreal injection, leading to blindness or even patient death.8
Viscous Drug Delivery
There is an increasing number of viscous formulations (>20 cP at room temperature) in the ophthalmology pipeline due to current trends in the pharma industry, such as the shift towards longer-acting drugs to reduce injection frequency and high-strength formulations. These drugs require higher injection forces, which can be problematic with manual intravitreal injections, due to the impact on user control, stability, speed of injection and other usability factors. The use of fine-gauge needles (30G or finer) further exacerbates the problems associated with high injection forces. Unfortunately, conventional approaches to mitigate this, such as using larger needle gauges and increased injection times, have limitations due to the unique circumstances of intravitreal delivery.
Injection Stroke Length
In a standard syringe, small doses (<50 μL) result in barely perceptible plunger travel. For example, only one millimetre of plunger movement in a standard 0.5 mL PFS and standard 1 mL long PFS results in the dispensing of 17 μL and 32 μL respectively. A Congruence user study showed that there is an optimal range for injection stroke length, starting above 4 mm (Figure 1). Injection stroke lengths below this can lead to major usability issues, such as uncertainty as to whether the dose was started or completed. In some cases, to avoid these issues, pharma companies may undergo expensive and lengthy reformulation efforts to increase the drug volume; however, this is limited by the maximum volume that can be safely injected into the eye.
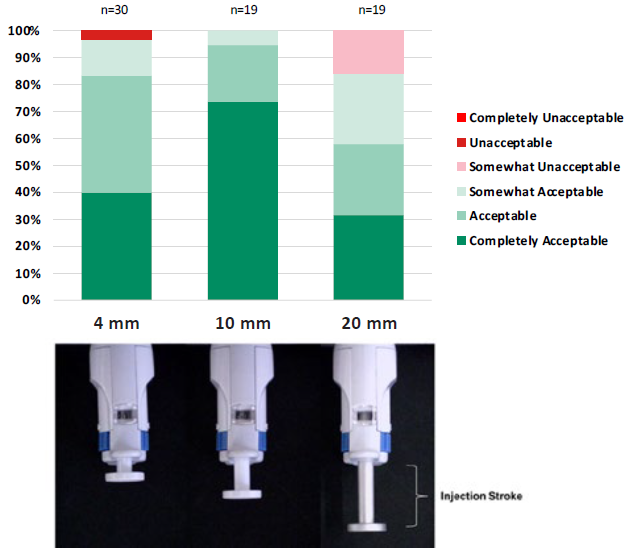
Figure 1: Acceptance scores at different injection stroke lengths, according to a Congruence user study.
ADDRESSING CHALLENGES IN INTRAVITREAL INJECTION
An option for addressing the challenges in intravitreal injection is to combine a silicone oil-free primary container designed for low-dose applications, such as Terumo’s PLAJEX™ ready-to-fill polymer syringe, and a mechanical, precision-dosing module, such as Congruence’s Microlitre Dosing Syringe (MDS) system, shown in Figure 2.

Figure 2: The Congruence MDS is comprised of Congruence’s microlitre dosing module and a PFS, such as the Terumo PLAJEX™ 0.5 mL shown here. It is delivered to end users fully assembled.
PLAJEX™ 0.5 mL Luer Lock Silicone Oil-Free PFS
PLAJEX™ 0.5 mL Luer lock Silicone oil-free PFS is specifically designed for challenging low-dose PFS products, making it suitable for high-value drug applications, such as intravitreal injection. In this way, PLAJEX™ enables pharmaceutical players to offer a safe, easy-to-use and effective treatment option for patients with ophthalmic disorders. The key advantages of the PLAJEX™ 0.5 mL Luer lock syringe include:
- Silicone oil-free
- Low subvisible particle level
- Tight dimensional tolerance
- Optional clear dose mark for dose confirmation.
Congruence MDS
The Congruence MDS is designed for applications involving a single manual injection of 5–150 μL. It is a disposable, single-use module that replaces the plunger rod and can be attached to any ISO 11040 compliant PFS. The MDS platform is versatile, with configuration choices that include:
- Pre-set dose (fixed dose, no user choice) or user-set dose (two to four dose volume options)
- Prefilled (syringe is primary drug container) or user filled (vial is primary drug container)
- The user-filled version enables the benefits of superior quality syringes to be made available for drugs where prefilling is not possible.
Congruence’s MDS was the recipient of the 2019 Drug Delivery Innovation Award from the Parenteral Drug Association. It can be used for intravitreal injections, as well as other intraocular injection routes, such as subretinal and suprachoroidal.
COMBINATION OF PLAJEX™ AND MDS AS A SINGLE SOLUTION
Silicone Oil-Free Syringe
Terumo’s proprietary i-coating™ technology eliminates the need for silicone oil in the syringe system, helping to prevent silicone oil being injected into patients. Terumo’s i-coating™ stopper provides consistent and predictable glide and injection forces (Figure 3), which can help control microlitre injections.
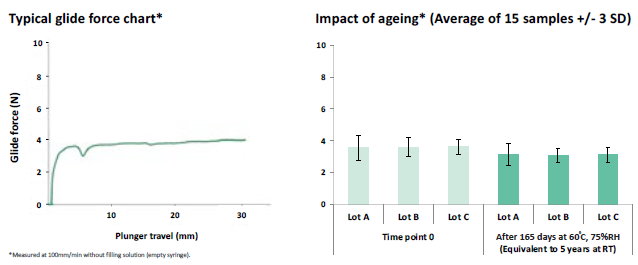
Figure 3: Terumo’s i-coating™ stoppers provide consistent and predictable break-loose and glide forces.
Low Sub-Visible Particle Count with Manufacturing Excellence
To enable pharmaceutical players to meet the stringent requirements of USP <789>, the PLAJEX™ 0.5 mL Luer lock syringe is supplied with extremely tight subvisible particle specifications (Figure 4). This is enabled by Terumo’s state-of-the-art manufacturing processes, which are fully automated to eliminate any human intervention that might introduce particles to the product.
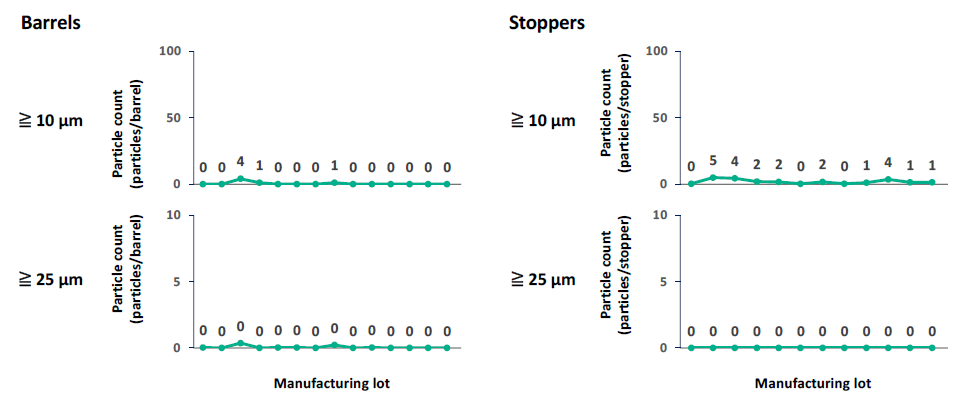
Figure 4: The PLAJEX™ 0.5 mL Luer lock syringe is supplied with extremely low subvisible particle level.
“With the Congruence MDS, instead of a 165 μL fill being required for a 50 μL dose, only 100 μL is needed – saving the equivalent of 1.3 doses.”
High Dose Accuracy
The use of a 0.5 mL syringe, such as Terumo’s PLAJEX™, instead of a 1 mL syringe, has been shown to improve the accuracy of delivering a 50 μL dose. However, this can be further improved by the use of the Congruence MDS device (Figure 5), which has the potential to have up to 25 times greater accuracy than an injection using a 1 mL PFS alone. The MDS has been shown to be able to deliver a dose as low as 5μL accurately (±2.5 μL).
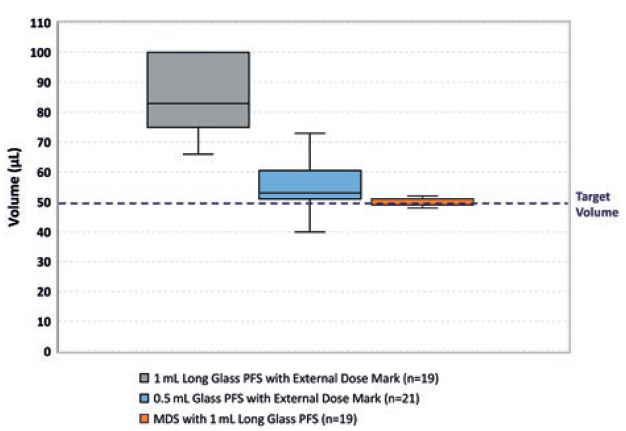
Figure 5: Congruence user study data showing the accuracy of delivering distilled water with a 1 mL long glass PFS, 0.5 mL glass PFS and MDS with 1 mL long glass syringe.
Pre-Assessment of Terminal Sterilisation Compatibility
There are various different technologies available for terminal sterilisation of a PFS, such as ethylene oxide (EO), vaporised hydrogen peroxide (VHP) and nitrogen dioxide (NO2). The impact of sterilisation on the stability of drug substances has to be carefully assessed at an early phase of the development to minimise project risks.
According to a study conducted with PLAJEX™, NO2 sterilisation exhibited essentially immeasurable NO2 ingress and no protein degradation, whereas denaturation of proteins was observed with EO and VHP sterilisation.9 The Congruence MDS has demonstrated suitability for sterilisation using E-beam, EO and NO2 methods.
Minimising Drug Waste
The first step to minimise drug overfill is to have a PFS with tight dimensional tolerances and low dead space. As per a Terumo study, the PLAJEX™ 0.5 mL Luer lock syringe has an inner diameter of 4.69–4.71 mm and residual volume of 10–15 μL (n=45).
A combination of the Congruence MDS with a 0.5 mL PFS was shown to require even less overfill. For example, with the Congruence MDS, instead of a 165 μL fill being required for a 50 μL dose, only 100 μL is needed – saving the equivalent of 1.3 doses. This is possible due to the controlled manipulation of the plunger stopper during priming and dose setting, along with low break-loose force.
Fewer Steps
PFSs involve fewer user steps than traditional syringe and vial solutions and so can help improve workflow efficiency. For example, Lucentis PFSs have been reported to have a 27–39% reduction in syringe preparation time10 and 57–63% time savings.11 These savings are possible with the Congruence MDS combined with a PFS. Therefore, while the Congruence MDS can be user-filled, prefilled versions convey an additional usability benefit.
Viscous Drug Delivery
A unique usability feature of the Congruence MDS, due to its inherent mechanical advantage, is the ability to deliver highly viscous drugs with up to a 75% reduction in the injection force experienced by the user, compared with a standard syringe alone, providing a significant usability benefit (Figure 6).
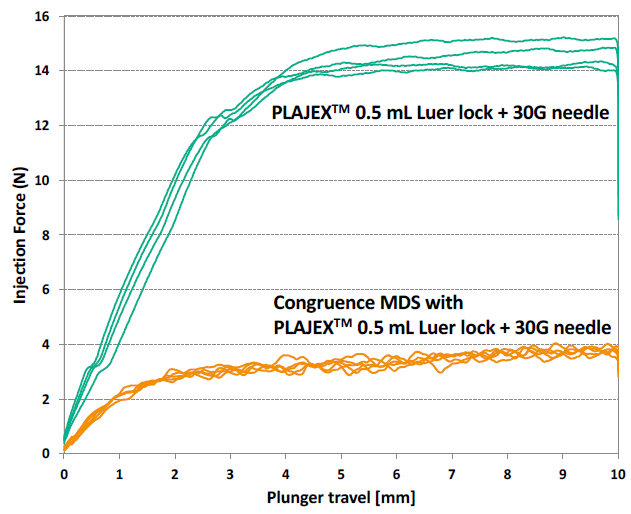
Figure 6: Comparison of injection force with and without Congruence MDS, injection force with and without Congruence.
Stroke Length
Due to the highly customisable design of the Congruence MDS, it also has the ability to accommodate the optimal stroke length for a given dose volume. This includes amplifying the injection stroke length, even for very small doses. For example, a dose volume of 50 μL in one MDS embodiment, using a 0.5 mL PFS, has a stroke length of 7.4 mm, compared with 2.9 mm for a PFS alone.
The improved performance of the Congruence MDS, as well as other features, such as controlled priming and air bubble removal in one simple step, translate into enhanced usability coupled with high user acceptance and preference. In a global study conducted by Congruence with 30 retina specialists, 100% of the respondents were able to perform all operation steps of the MDS in an error-free manner. Additionally, 87% of the respondents said that they would prefer to use the prefilled Congruence MDS over other currently available delivery options.
CONCLUSION
Novel drug delivery solutions can add tremendous value to intravitreal injection applications due to the unique challenges for this injection method. The combination of Terumo’s PLAJEX™, a silicone oil-free syringe with tight tolerances designed for low-dose applications, and Congruence’s MDS, a mechanical, manual injection device for a better dosing experience, has many advantages, especially when accuracy and precision is necessary, such as for doses of 50 μL or less, and for drugs requiring high injection forces.
REFERENCES
- Rimpelä AK et al, “Pharmacokinetic Simulations of Intravitreal Biologicals: Aspects of Drug Delivery to the Posterior and Anterior Segments”. Pharmaceutics, 2018, Vol 11(1), p 9.
- Baker C et al, “Designing the Ranibizumab Prefilled Syringe for Optimal Safety and Efficiency”. ASRS Annual Meeting 2017 poster presentation, Aug 2017.
- Khurana RN, Chang LK, Porco TC, “Incidence of Presumed Silicone Oil Droplets in the Vitreous Cavity After Intravitreal Bevacizumab Injection With Insulin Syringes”. JAMA Ophthalmaol, 2017, Vol 135(7), pp 800–803.
- Olea JL et al, “Silicone oil droplets in repackaged anti-vascular endothelial growth factors for intravitreal injections: In search of the main source of contamination”. Eur J Ophthalmol, 2020, Vol 30(4), pp 774–779.
- Koshino K et al, “Functional evaluation and characterization of a newly developed silicone oil-free prefillable syringe system”. J Pharm Sci, 2014, Vol 103(5), pp 1520–1528.
- Loewenstein I et al, “Accuracy and Precision of Intravitreal Injections of Anti-Vascular Endothelial Growth Factor Agents in Real Life: What Is Actually in the Syringe?”. Retina,2019, Vol 39(7), pp 1385–1391.
- Meyer CH et al, “Accuracy, precision and repeatability in preparing the intravitreal dose with a 1.0-cc syringe”. Acta Ophthalmol, 2012, Vol 90(2), pp e165–e166.
- Storey PP et al, “The Impact of Prefilled Syringes on Endophthalmitis Following Intravitreal Injection of Ranibizumab”. Am J Ophthalmol, 2019, Vol 199, pp 200–208.
- Fujiwara S, “Chemical-gas Sterilization of External Surface of Polymer-based Prefilled Syringes and Its Effect on Stability of Model Therapeutic Protein”. J Pharm Sci, 2022, Vol 111(1), pp 41–50.
- Souied E et al, “Ranibizumab prefilled syringes: benefits of reduced syringe preparation times and less complex preparation procedures”. Eur J Ophthalmol, 2015, Vol 25(6), pp 529–534.
- Subhi Y, Kjer B, Munch IC, “Prefilled syringes for intravitreal injection reduce preparation time”. Dan Med J, 2016, Vol 63(4), A5214.

