To Issue 130
Citation: Kraus R, “The Demand for Metered Dose Eye Drops: A Dose Delivery Study”. ONdrugDelivery, Issue 130 (Mar 2022), pp 11–14.
Rouven Kraus discusses a dose delivery study comparing various delivery technologies designed to meet the demand for metered dose eye drops.
The conjunctival sac of the human eye holds 7–10 μL of tears and has an overall capacity of 30 μL of fluid at a time.1 To apply the right dose of medicine to the eye, drug delivery companies designed eye-drop devices that apply appropriate doses of 28–50 μL. Prescription drugs (e.g. prostaglandins for glaucoma treatment) usually come in delivery devices applying a dose of about 30 μL. The active ingredient is kept in the eye without any fluid spilling out. Moisturising eye drops containing, for example, sodium hyaluronate use dropper devices with a dose of about 30 μL or even about 50 μL if the product is intended to flush the eye. Especially for expensive APIs, drug manufacturers should turn their attention to prevent waste of the active and to avoid any overdosing risks. To do so, they are faced with using metered dose delivery technologies.
“Metered dose dropper pumps are unique in this field as they are the only preservative-free technology that is based on a purely mechanical pump system.”
There are a multitude of different delivery technologies available on the market. Single-dose vials are designed to dispense a single amount of medicine and then will be discarded. Three-piece droppers are conventional multidose bottles for preserved medications. Regulatory bodies recommend the use of preservative-free delivery technologies to protect the ocular surfaces from any side effects caused by preservatives such as benzalkonium chloride. Squeeze dispensers (such as Aptar Pharma’s OSD System or Nemera’s Novelia®) are such preservative-free multidose bottles that protect the container content from microbial impurity.
Metered dose dropper pumps (such as Aero Pump’s 3K® pump or Ursapharm’s (Saarbrücken, Germany) COMOD® system) are unique in this field as they are the only preservative-free technology that is based on a purely mechanical pump system. They always offer an accurate dose delivery with just one drop delivery per activation (Figure 1).
Figure 1: Precise dose delivery of a metered dose pump.
Aero Pump conducted a dosage size determination study with various delivery technologies that are available on the market. The study included several multidose eye-drop bottles.
The assay is based on the dose-uniformity requirements as set forth in the relevant pharmacopoeial chapters (USP Chapter <905> Uniformity of Dosage Units and Ph Eur <0676> Nasal Preparations). Ten samples per device are assayed individually by using calibrated balances. The whole study is performed by a single trained person to avoid any fluctuations. All devices are filled with a lubricating ophthalmic formulation available on the market. The assay is carried out on the amount of solution that is removed from an individual container in conditions of normal use, whereas the first 20 doses are discharged until a consistent dosage is achieved. For some devices it is necessary to prime the system to release the air from the device. The results are expressed as the delivered dosage units. Therefore, pharmacopoeias foresee the following dose uniformity limits:

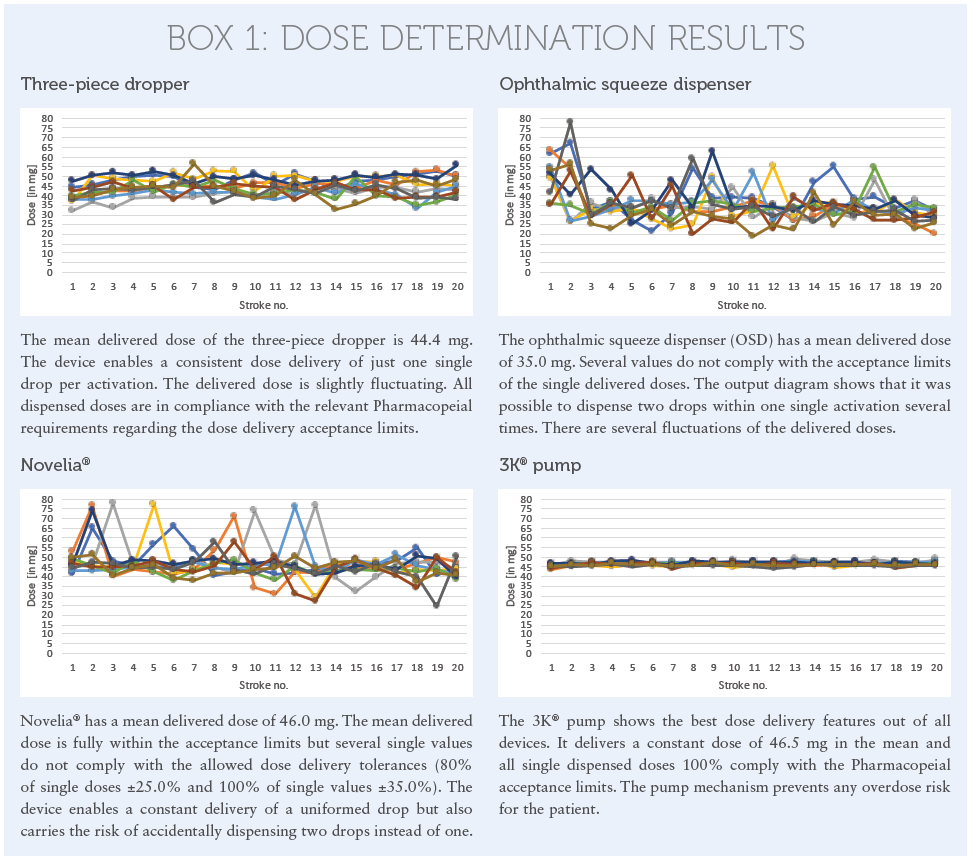
The results of this dose delivery assay are shown in the diagrams in Box 1.
“The results show that the pump-based mechanism used in the
3K® system achieved the best RSD value.”
It is shown that all devices show good dose-uniformity results. But it is clearly visible that, except for the pump-based mechanism used in the 3K® technology, all other devices carry the risk of dispensing two drops per activation instead of just one. If the patient squeezes the bottle, they might in still two drops in their eye with the doubled active content. With some squeeze dispensers there is even the potential to create an extremely uncomfortable jet in the worst-case scenario.
The pump mechanism used in the 3K® pump avoids such overdosing reaction since the pump creates one defined dose per each activation, drop by drop (Figure 2).
Figure 2: The risk of overdosing the eye.
During the assay the different devices were also compared with the relative standard deviation (RSD) by using the following formula:
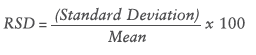
The RSD value determines the deviation of all delivered drops in relation to the mean dispensed dose. The less the RSD rate, the more constant the dose uniformity. Table 1 shows the RSD results of all devices.
| Device | Relative Standard Deviation |
| Three-piece dropper |
10.5% |
| OSD | 26.5% |
| Novelia® | 17.1% |
| 3K® | 2.0% |
Table 1: The RSD of every tested device.
“The pump always works with the same activation force, which remains stable until the bottle is entirely emptied.”
The results show that the pump based mechanism used in the 3K® system achieved the best RSD value. All doses only fluctuate in a ratio of 2.0%. The minimum delivered dose out of all values is 43.5 mg and the maximum dose is 49.1 mg. The tested squeeze devices are more fluctuating in the dose uniformity. This is because the squeeze mechanism is controlled by pressure. Different pressure applied on the bottle may influence the delivered dose. And this is, in fact, the reality when looking at elderly patients with dexterity issues who may have less power to properly squeeze out the drops. Other patients may apply too much pressure and will squeeze out a higher dose – up to a doubled dose. Whereas the pump always works with the same activation force, which remains stable until the bottle is entirely emptied. This is user independent.
From a previous usability study, one third of subjects complained that “more than one drop came out”. Improper delivery of drugs can lead to treatment failure, especially in the case of glaucoma patients. It increases the risk of potential side effects, such as burning or stinging, high blood pressure, fatigue, irregular heart rate, etc.
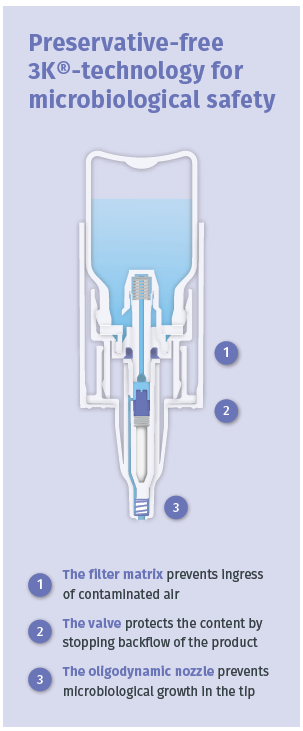
Figure 3: The principle of the 3K® technology with its triple protection barriers against microbiological contamination.
The 3K® pump ophthalmic system is designed in a way that the product chamber determines the output of the device. For a defined output of 45 mg, the pump will only release a drop size of 45 mg. The product chambers will be filled with the fluid again from the next activation of the pump. The 3K® pump eyedropper is also available in lower dosage sizes such as 28 mg. The principle of the pump-based mechanism simply avoids overdosing.
ABOUT AERO PUMP’S PRESERVATIVE-FREE OPHTHALMIC MULTIDOSE SYSTEM
Aero Pump has developed a preservative free multidose system with 3K® technology for ocular delivery. Special germ-reducing components inside the 3K® system ensure the microbiological safety of the device (Figure 3).
The pump system is available for use with plastic or glass containers in various fill sizes and, in terms of reducing container interaction with the product, this is a particular advantage. The 3K® system delivers an accurate dose over the whole lifecycle of the product, with one measured drop per actuation. The actuation force of Aero Pump’s ophthalmic multidose system is stable, independent of the residual liquid inside the container.
Alongside the development of the ophthalmic multidose devices, Aero Pump has developed various customer-friendly actuation aids that enable a convenient application of the drop into the eye of the patient (Figure 4).
REFERENCE
- Djebli N et al, “Ocular Drug Distribution After Topical Administration: Population Pharmacokinetic Model in Rabbits”. Eur J Drug Metab Pharm, 2016, Vol 42(1), pp 59–68.

