Citation: Clayton J, “3D Printing Drugs: Learning from the Pioneers”. ONdrugDelivery Magazine, Issue 99 (Aug 2019), pp 12-15.
Jamie Clayton reviews the potential of 3D printing as a pharmaceutical production method and looks at the associated implications for formulation development, focusing on powder-based printing. He examines what leading experts now understand about how to specify powder feeds for 3D printing, referencing experimental data, and highlights the importance of powder-flow characterisation in this context.
In August 2015, the US FDA approved SPRITAM®, the first 3D-printed drug, for the treatment of seizures in adults and children with epilepsy. Manufactured on a platform that traces its heritage back to technology originally licensed from MIT (Cambridge, MA, US), this approval is useful in providing clear evidence of the possible benefits of printing drugs. These include the potential to deliver very high drug loadings and to produce highly porous tablets that disintegrate rapidly on contact with minimal amounts of water, and successfully delivering drugs to patients who have dysphagia (difficulty swallowing) and/or struggle with conventional tablets.
“The vision of pharma manufacturing potentially enabled by 3D printing is one in which pharmacists will ultimately switch from dispensing uniform, ready-made products to printing drugs to order, accounting for factors such as genetics, age, gender, and biochemical and disease profile.1,2”
Beyond these established benefits, the industry continues to debate and explore the possible long-term role of 3D printing in the delivery of personalised medicine, for example, and in localised manufacture. 3D printing offers exciting flexibility to tailor the size, drug-release profile and shape of oral solid-dosage forms, a defining generic benefit of the technology being the minimal cost of bespoke manufacture. Once a printer is in place, making a new product can be as simple as switching raw ingredients and selecting the required operating protocol.
The core attractions of 3D printing are obviously not uniquely interesting to the pharmaceutical industry; in fact, other sectors are more advanced in embracing this innovative technology. 3D printing is now well established for finished part production in the aerospace and automotive sectors and, more relevantly, is widely used in medical engineering – for example, for the construction of artificial bones and dental implants. As the pharmaceutical industry begins its exploitation of 3D printing, it seems sensible to consider what can be learned to accelerate progress.
EXPLORING THE POTENTIAL OF 3D PRINTING
The vision of pharma manufacturing potentially enabled by 3D printing is one in which pharmacists will ultimately switch from dispensing uniform, ready-made products to printing drugs to order, accounting for factors such as genetics, age, gender, and biochemical and disease profile.1,2 Such a transformation would present significant regulatory challenges but, at the same time, it offers substantial opportunities to tailor therapeutic regimes cost effectively to increasingly small population groups – for example, to treat paediatrics and/or geriatrics more effectively and to tackle orphan diseases. Personalisation is the ultimate endpoint of such a process and could significantly enhance clinical outcomes, even with existing drugs.
On-demand prescription printing would reduce the need for products with extended shelf life. It also offers opportunities to improve patient compliance (the so called “pill burden”) through the use of polypills – dosage forms containing multiple actives that are printed to individual patient requirements. For developed economies, 3D printing has potential as a highly efficient,agile platform for domestic production, to reduce exposure to geopolitical risk and supply chain disruption, while for remote, poorly connected communities it provides relatively low-cost access to high-quality, well-manufactured drugs.
So, how close are we to realising this vision?
The approval of Aprecia Pharmaceuticals’ (Blue Ash, OH, US) SPRITAM® is undoubtedly an important milestone and the ZipDose technology that underpins it is now being more generally promoted for the rapid delivery of high drug loads and/or multiple APIs. Dose sizes are two to three times higher than can be delivered via conventional orally disintegrating tablet (ODT) technology, dispersion times are generally faster and the technology offers considerable versatility for taste masking. The potential for brand extension provides a stimulus for technology uptake.3
With respect to on-demand printing, FabRx – a spin-off company from University College London – has recently been awarded funding to develop the world’s first 3D printer for personalised medicines. The aim is to develop a printer that will be safe and fit for purpose to produce printed tablets (“printlets”) in a hospital pharmacy setting. Work on patient response to 3D-printed tablets is also underway, with the world’s first paediatric trial currently taking place at Alder Hey Hospital (Liverpool, UK). Within two years this team plans to transition from testing placebo products to those containing an active pharmaceutical ingredient (API), focusing on hydrocortisone which is currently dosed in poorly controlled levels because of the need to break up pills to give children a weight-related dose.2
“One area of focus is the identification of critical material attributes for excipients for 3D printing and the development of correlations between material attributes and product performance.”
On the regulatory front, the US FDA’s Center for Drug Evaluation and Research (CDER) is actively engaged in research to address questions raised by using 3D printing specifically for drug products, recognising that progress in this area will be crucial.4 One area of focus is the identification of critical material attributes for excipients for 3D printing and the development of correlations between material attributes and product performance. This is a major focus for those working to exploit the technology commercially too; optimising pharmaceutical formulations for printing is a new challenge.
AN INTRODUCTION TO 3D PRINTING TECHNOLOGY
Technologies that can be used to print pharmaceuticals include material extrusion processes such as semi-solid extrusion, which is suitable for printing gels or pastes, and fused deposition modelling – the construction of products from a pharmaceutical-grade polymer filament. Stereolithography, a process from the vat photopolymerisation family, can also be applied. This involves using a laser to cure layers of liquid polymer, with API incorporated into the emerging polymeric network. The focus of this paper is powder-based processes such as binder jetting – the powder-liquid technology originally developed by MIT – and powder-bed fusion (PBF), alternatively known as selective laser sintering. These both involve the joining of successive layers of powder to construct the finished dosage form (Figure 1).
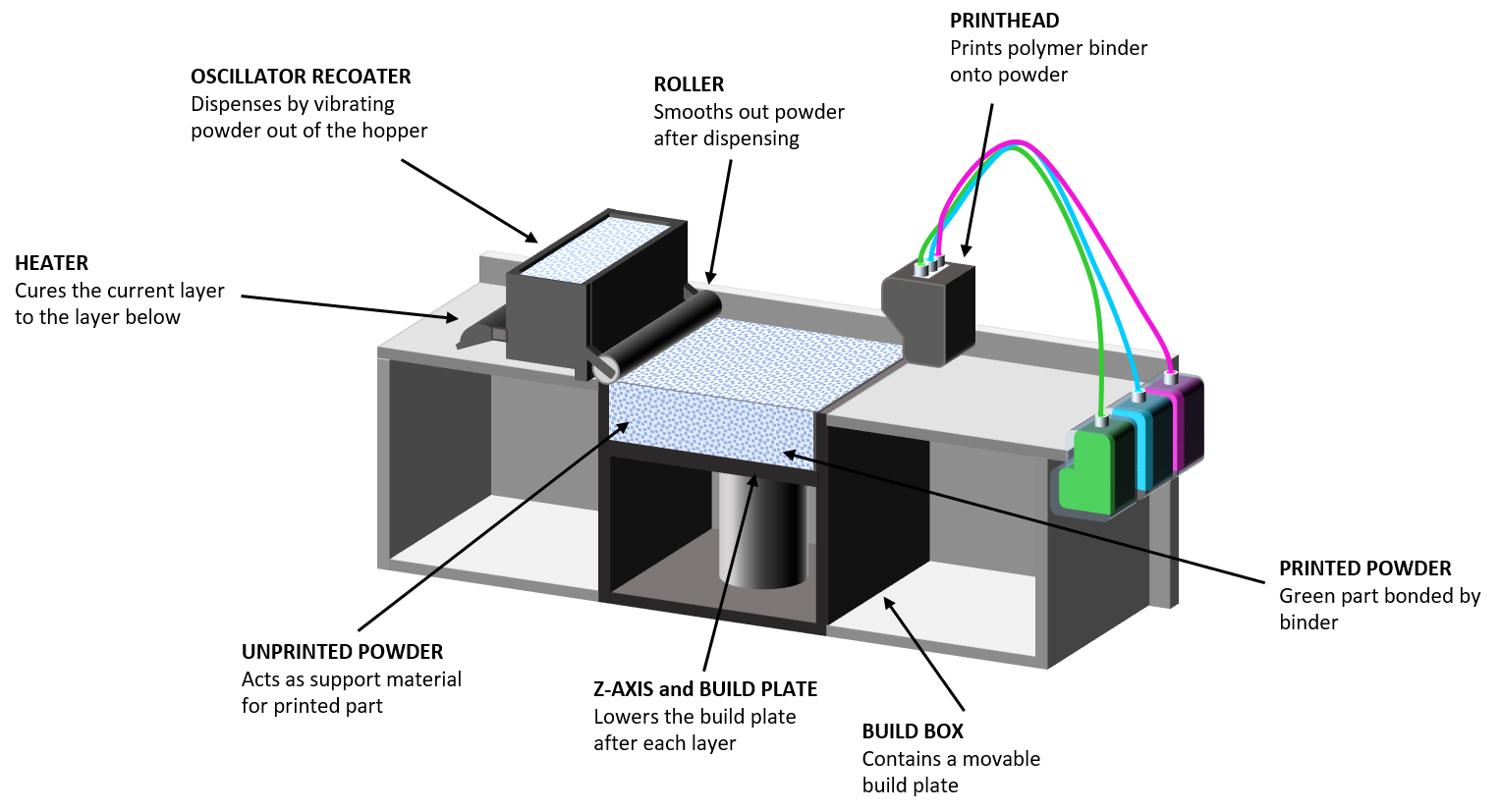
Figure 1: Schematic of a binder jetting process which involves the rapid spreading of powder layers just tens of microns thick.
In binder jetting and PBF processes, the formulation is delivered or spread across a build surface in layers just tens of microns thick. With binder jetting, a print-head then releases droplets of liquid/polymeric binder into the powder bed, which are thermally cured to bind defined areas, progressively building the dosage form layer by layer. PBF processes are strictly analogous but powder layers are fused through the application of heat, using a laser, which obviously has implications with respect to the protection of thermally labile drug substances; curing is a lower temperature, far less energy intensive process.
FORMULATION CHARACTERISATION: FOCUSING ON FLOWABILITY
When characterising pharmaceutical formulations for 3D printing, there are lessons to be drawn from experience of conventional tableting processes and from those already applying 3D printing in other sectors. This is especially true with respect to the best methods to apply for bulk powder testing, specifically the measurement of powder flowability – a property of defining importance in both processes.
In a conventional direct compression tableting process, raw ingredients are dispensed from feed hoppers at a consistent, closely controlled flow rate into the mixing stages that precede granulation, where used, or directly into the feed frame. In a hopper, raw ingredients are subject to compressive storage under their own weight, resulting in moderate stress at the discharge outlet. Granulation of a tableting blend is extremely common, to prevent segregation and improve flow properties, with flow additives routinely incorporated to further enhance flowability throughout the process.
Powder flows from the feed frame in a relatively loosely packed, low stress state and is swept into the dies to ensure a complete fill. This sweeping action can exert an element of forced flow, with blades pushing the powder into the confined die space. Air can have a lubricating, enhancing effect on powder flowability but uniform filling, to a defined bulk density, calls for a formulation that settles rapidly, easily releasing entrained air. The final step of conventional tableting is compression followed by ejection from the die.
Shear cell testing was developed specifically to quantify powder properties for hopper design and remains valuable for investigating behaviour under the moderate stress that prevails in storage. However, flow behaviour in other areas of the traditional tableting process are more successfully predicted by dynamic testing5 (see Box 1). Basic flowability energy (BFE), for example, is highly relevant for rationalising performance in the feed frame, while measurements of AE valuably quantify how flowability changes with air content. Instrumentation for dynamic testing also measures shear and bulk powder properties, such as permeability and compressibility, which characterise the ease with which the formulation releases entrained air and its response to compression, respectively. As a result, it is able to provide the multi-faceted insight needed to optimise tableting processes.
The initial feeding of raw ingredients from a feed hopper into a 3D printing process is similar to a conventional process, so shear data remain relevant. However, the powder then transitions into a low stress environment with high flowability under such conditions essential for the sequential deposition of powder layers. Indeed, in 3D printing, processing rate is effectively defined by the ability of the powder to consistently form even layers of defined thickness; dynamic flow properties are valuable for defining performance with respect to this crucial aspect of behaviour. As with die filling, the goal is a layer with minimal or controlled voidage since voids inhibit fusing/binding, ultimately impacting the mechanical integrity of the finished product; bulk density measurements can be helpful in rationalising packing behaviour.
An important point to appreciate about 3D printing is that only a proportion of the powder in a given layer is bound into the finished product; powder recycling is therefore vital for sustainable manufacture. This makes the stability of 3D formulations an important issue. The characterisation requirement is to understand the behaviour of both fresh/virgin and recycled material, with properties potentially changed by the printing process. Dynamic test protocols for stability can be extremely useful in this regard.
BOX 1: AN INTRODUCTION TO DYNAMIC POWDER TESTING
Dynamic powder properties are determined from measurements of the axial and rotational forces acting on a blade, or impeller, as it is precisely rotated through a powder sample (see image). The technique was developed specifically to measure powder flowability under conditions that simulate the process environment and powders can be measured in a consolidated, moderate stress, aerated or fluidised state. This ability to tailor the test environment, particularly with respect to direct assessment of the impact of air, sets dynamic testing apart from traditional USP methods. Furthermore,
the technique offers exemplary repeatability, reproducibility and sensitivity.
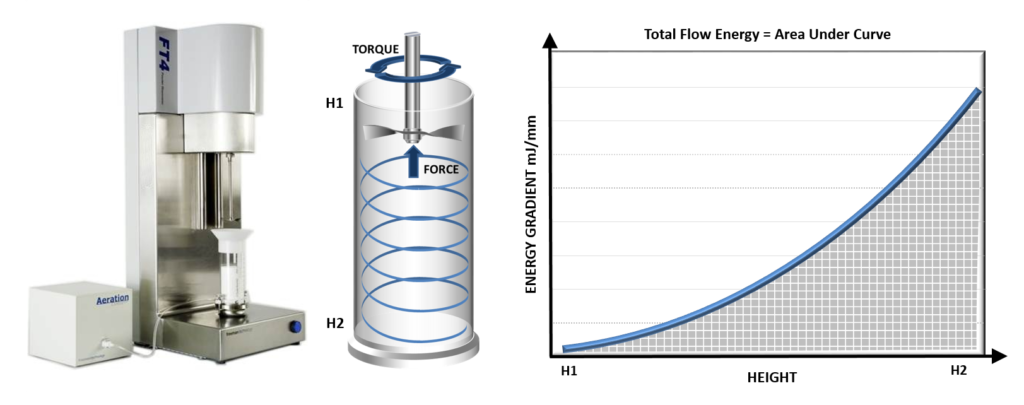
Dynamic testing measures the powder in motion and can be applied to samples in a consolidated, moderate stress, aerated or even fluidised state.
Dynamic properties include:
- Basic flowability energy (BFE) which quantifies confined flow behaviour (forced flow) in a low-stress powder and is measured during a downward traverse of the blade
- Specific energy (SE) which quantifies the unconfined flow properties (gravity flow) of a powder in a low-stress state and is measured by rotating the blade upwards through the sample
- Flow rate index (FRI) which quantifies sensitivity to flow rate and is determined from measurements of BFE at different blade speeds
- Stability index (SI) which quantifies physical stability and is determined from repeat measurements of BFE on the same sample
- Aeration energy (AE) which quantifies the impact of air on flowability via measurements of BFE at a defined air-flow rate through the sample.
KNOWLEDGE TRANSFER
Reviewing practice in industries in which 3D printing is more commercially established is illuminating in terms of the specifications already in place to identify powders that will print successfully. ExOne (Pittsburgh, PA, US) is a global leader in binder jetting technology. In 1996, the company obtained the licence for the 3D-printing process developed at MIT for metal and sand parts and it has since gone on to develop and commercialise technology with applications in the automotive, aerospace, heavy industry and energy sectors.
Over several years, ExOne has progressively refined the specificationapplied to differentiate powders that will print from those that will not and the parameters routinely measured now include:
- Particle size and size distribution (Dv10, Dv50 and Dv90)
- Particle morphology (shape)
- Stability index (SI)
- Flow rate index (FRI)
- Cohesion
- Wall friction angle
- Permeability
- Compressibility.
Tests are also carried out to assess binder compatibility.
Particle size and size distribution impact the flowability and packing behaviour of powders, as does particle shape; more regularly shaped particles are typically preferred for 3D printing because of their enhanced fluidity and packing efficiency. However, experience has shown that these parameters alone cannot identify powders that will perform acceptably in the company’s printers. The remaining parameters are bulk powder properties – dynamic (SI and FRI), shear (cohesion and wall friction angle) and bulk (permeability and compressibility) – all now measured for each new powder using dynamic powder testing (Table 1 & 2).
| Powder A | Powder B | |
| D10 (μm) | 6.4 | 6.6 |
| D50 (μm) | 16.1 | 15.8 |
| D90 (μm) | 29.1 | 30.0 |
Table 1: Data for two alternative supplies, illustrating the strength and application of the powder specification. Powder B, an alternative supply, is less expensive than Powder A; testing was being carried out to determine whether a switch could be made to enhance profitability.
| Minimum | Ideal Case | Maximum | Powder A | Powder B | |
| Stability Index | 0.65 | 1.00 | 1.2 | 1.03 | 1.52 |
| Flow Rate Index | 0.96 | 1.00 | 2.50 | 1.35 | 1.27 |
| Cohesion (kPa) | 0.11 | low | 1.76 | 0.62 | 0.62 |
| Wall Friction Angle (°) | 10.40 | Low | 32.10 | 27.80 | 24.02 |
Table 2: Particle size data for Powder A and B is similar but a high SI correctly identifies the alternative powder as being unsuitable.
Powder A and B have closely similar particle size distributions and scanning electron microscopy revealed comparable morphology (data not shown). In fact, the powders were only substantially differentiated in terms of SI, which at 1.52 was much higher for Powder B, outside the upper limit of 1.20. This figure suggests that the powder is physically unstable and in print runs the impact of this instability became clear. While Powder B initially performed well, the quality of printed parts gradually degraded over time to the extent that after 4–5 cycles it became impossible to print successfully. Further investigation ultimately attributed this behaviour to the presence of flaky particles becoming prone to interlocking and, by extension, poor flowability with re-use.
Experience at ExOne indicates that flowability data are critical in terms of defining the printability of powders and, as with conventional tableting, dynamic, shear and bulk properties are all relevant. Given the similarity of powder-liquid 3D-printing processes for pharmaceutical applications, it appears highly likely that this is a transferrable learning and that formulations for printing will also need to be specified with reference to all three types of properties.
CONCLUSION
3D printing holds considerable promise for the pharmaceutical industry. While early wins include the ability to deliver high dosages in rapidly dissolving tablets that ease the administration of drugs to patients facing difficulties with conventional oral solid dosage forms, the longer-term picture is transformative. Personalised drug products, containing a unique combination of active ingredients, printed to order at a unique dosage, in a local hospital or pharmacy, could be an achievable reality.
Those exploiting powder-based 3D printing technologies in other sectors have already discovered that such processes call for formulations with exemplary flowability and the measurement of dynamic powder properties has proven critical in differentiating powder that will print successfully. Such experience indicates that the application of effective bulk powder testing strategies will be essential to characterise pharmaceutical formulations for printing and realise the full potential of this exciting technology.
REFERENCES
- Huang S, Huang J, “3D Printing Drugs: more precise, more personalised”. Pharma Times, Jan 2018.
- Cave H, “3D printing could give you a better pill to swallow”. Mosaic (Wellcome), Jan 2019.
- Wetherhold D et al, “Redefining fast melt for pharma: Achieving high drug load with rapid dispersion using 3D printing”. Aprecia White Paper.
- Zidan A, “CDER researchers explore the promise and potential of 3D printed pharmaceuticals”. Spotlight on CDER Science.
- Freeman T, “Characterising powders for better solids processing”. Internal Publication, Freeman Technology, May/Jun 2010.

