Citation: Rafiei R, “Macro Trends Accelerating the Adoption of Connectivity in Drug Delivery”. ONdrugDelivery Magazine, Issue 98 (Jun 2019), pp 24-28.
Ramin Rafiei argues that solely relying on the clinical efficacy of medications is no longer adequate for improving patient clinical outcomes. Connected therapeutics – the augmentation of drugs through sensors and connectivity – are now a clinical source for real-world data and provide an opportunity to bridge the efficacy-to-effectiveness gap. This next frontier in drug delivery, powered by connected therapeutics, will be data driven, personalised, outcomes based and accessible.
Over the past three decades, SHL Group has partnered with the majority of the world’s top 25 leading pharmaceutical and biotech companies to bring more than 30 autoinjector-drug combination products to market. Autoinjectors represent one of the fastest growing segments in drug self-administration, supporting a range of chronic conditions including diabetes, rheumatoid arthritis, multiple sclerosis, migraine and cardiovascular disease.
“Chronic conditions
place healthcare systems
worldwide under
significant stress.”
The increasing prevalence of chronic conditions has amplified the demand for new medication options.1 As a result, the biopharmaceutical market is growing at double the rate of traditional small molecule pharma.2 To cater for the growing pipeline of these macromolecule biologics and biosimilars, SHL continues to invest heavily in the development of drug delivery devices, covering a wide range in both volume and viscosity (Figure 1)
EFFICACY TO EFFECTIVENESS
Chronic conditions place healthcare systems worldwide under significant stress. For example, in the US, the number of people with diabetes has grown 20-fold over the past three decades,3 while costs on a per-patient level have also increased by 7.5% annually.4 Unlike any other industry, where cost goes down as products and services scale, there is both an increasing prevalence of chronic conditions and increasing cost for managing each patient.
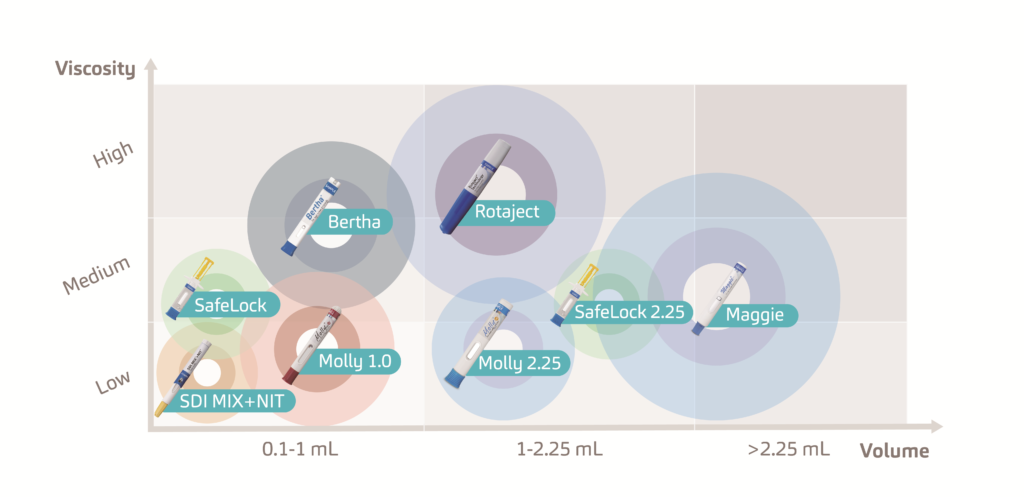
Figure 1: SHL Group develops a range of disposable and re-usable injectors that can accommodate high volumes and high viscosities and can be enhanced through digital implementations.
“Clinical trials take place in highly controlled environments whereas, in the real world, the patient needs to self-manage their treatment … Solely relying on the clinical efficacy of new medications is not
the solution to chronic disease management.”
A major reason for this trend is the efficacy-to-effectiveness (E2E) gap. Figure 2 illustrates the E2E gap for Type 2 diabetes. The top line shows the 26 new Type 2 diabetes medications approved between 1999 and 2014, each with proven clinical efficacy.5 The bottom line shows the percentage of the population with Type 2 diabetes which has the condition under control, specifically defined as a haemoglobin A1c level below 7%. Diabetes patients typically monitor their glucose levels through a mechanism known as the A1c test, which is the average blood sugar level for the past two to three months. A1c levels are an indication of treatment efficacy, therefore, patients are tasked to keep their A1c levels as close as possible to their target percentages, which are typically around 6-7%.6 While between 1999 and 2003 new therapies, such as Sanofi’s Lantus (insulin glargine) proved effective in helping patients reach optimum A1c levels, this trend failed to continue beyond 2003.7
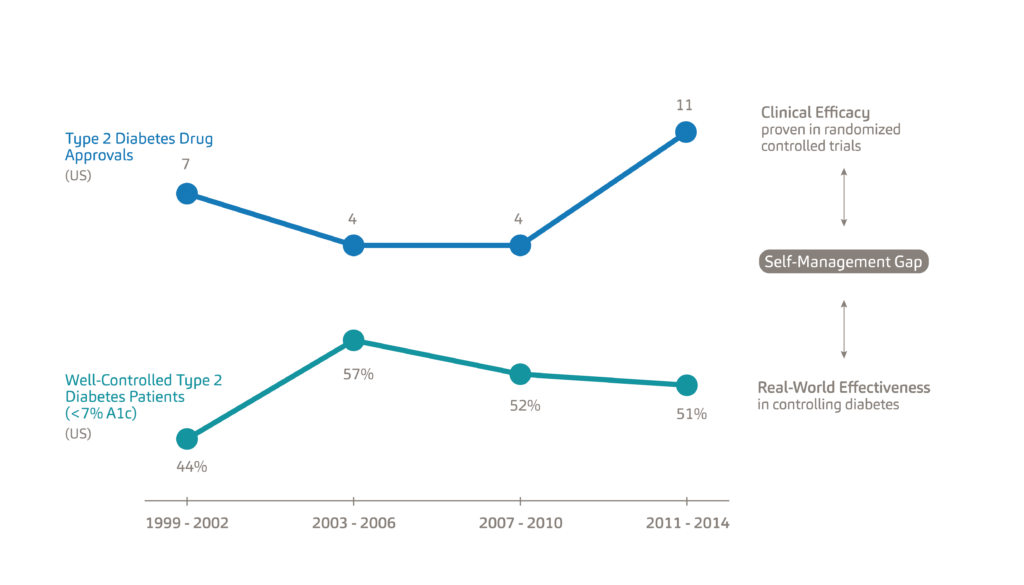
Figure 2: An illustration of the efficacy-to-effectiveness gap for Type 2 diabetes.
The E2E gap refers to the separation between the clinical efficacy of new drugs as determined by randomised controlled trials and their real-world effectiveness. Given the intense frequency of follow-up and support for participants, clinical trials take place in highly controlled environments whereas, in the real world, the patient needs to self-manage their treatment. As a result, solely relying on the clinical efficacy of new medications is not the solution to chronic disease management; rather, we need to find ways of helping patients manage existing conditions – what we refer to as the self-management gap.
Bridging this gap demands a systemic focus on medication adherence as typically 50% of people with chronic conditions fail to take their medications as prescribed.8 The consequences of poor adherence to long-term therapies are poor health outcomes and increased healthcare costs. Non-adherence to medication, however, is a complex and multidimensional healthcare problem and stems from the diversity of patient behaviours and barriers.8 The fact that non-adherence remains a major problem after decades of research shows just how difficult it can be to identify and address effectively.
It has been shown that non-adherent patients cost the healthcare system significantly more in medical costs across all chronic disease states.9-12 Today, the measured adherence level of 50% or lower across most chronic conditions is typically based on prescription refill claims data.13-16 It has also been shown that true adherence or dose-level adherence is 15-30% lower when measured with connected drug delivery devices.17-18 This means the reported avoidable medical costs associated with medication non-adherence are actually underestimations since patients considered adherent based on prescription refill claims data might not be taking the drug as prescribed.9-12
BRIDGING THE GAP
“Once we are able to
measure adherence,
we can address it through
interventions, support
and resources.”
Connected therapeutics – the augmentation of drugs through sensors and connectivity – generate real-world data. This data provides a digital representation of the patient’s true self-care behaviours and health outcomes outside the clinic. Real-world data from connected therapeutics also complements other traditional sources of clinical data, including electronic medical records as well as pharmacy and medical claims. The fusion of these data sets can now provide an unparalleled view of patient behaviours not formerly possible.
Drug delivery devices, which passively and objectively measure adherence at the point the patient is interacting with their medication, provide the greatest opportunity to bridge the E2E gap. Once we are able to measure adherence, we can address it through interventions, support and resources. If we are able to improve adherence, then we will also indirectly improve both clinical and financial outcomes. Today, Moore’s law, the alignment of incentives among stakeholders, and improved regulatory pathways, are converging to pave the way for connected therapeutics.
ACCELERATION BY TECHNOLOGY
In the biopharmaceutical industry, Eroom’s law (Moore spelt backwards) refers to the exponentially increasing cost of bringing new drugs to market.19,20 On the other hand, Moore’s law has transformed computing through exponentially decreased costs. As technology continues to get smaller, lighter, more efficient and easier to use, sensors and computers are making their way into drug delivery devices. Here, injectable therapies are still in their infancy when compared with connected respiratory and oral therapies. However, studies across the three connected therapeutic categories have consistently demonstrated improved patient adherence and overall health outcomes.21-24 Therefore, while bringing drugs to market with proven clinical efficacy is an increasingly costly endeavour based on Eroom’s law, the application of Moore’s law to drug delivery has the potential to improve the return on investment by bridging the E2E gap.
ACCELERATION BY ALIGNMENT
The second and most important trend driving the adoption of connectivity in healthcare is the alignment of incentives among stakeholders around quality and outcomes. Pharmaceutical companies are increasingly taking on risks through value-based contracts as a way to get more coverage and improve their formulary positions.25-26 Under these contracts, the price that insurers pay for drugs is tied to either the clinical outcome or an adherence threshold. Interestingly, adherence is a common factor in all the contracts, either as a qualifying event (i.e. only patients who refill their prescription on time are included in the contract) or as an outcome(i.e. the pharmaceutical company is paid less for patients who are non-adherent). Connected drug delivery devices, which generate dose-level adherence data, will become an important tool for pharmaceutical companies in these value-based contracts. Dose-level data will limit exposure when adherence is a qualifier for the contract by identifying patients who refilled their prescription but are not using the medication properly.
The US 21st Century Cures Act of 2016 (Cures Act), signed into law on December 13, 2016, is designed to accelerate the discovery, development and delivery of new cures and treatments for disease.27 The Cures Act has made it possible for pharmaceutical companies to expand their label into new conditions using real-world data instead of running additional clinical trials for each condition. Since we know real-world adherence is low, connectivity in drug delivery devices will be a necessary tool that allows pharmaceutical companies to generate the evidence from cohorts of patients who are using the medication as prescribed.
Healthcare providers are another important stakeholder driving the adoption of connectivity. Historically, providers have not been on board with remote monitoring primarily due to the absence of a financial incentive. With the 2019 US Proposed Rule, remote care enabled by connected devices has moved to the forefront and providers are being reimbursed for high-quality, high-impact care.28 New Medicare codes now reimburse physicians for remote patient monitoring devices and services. Given there are over 55 million Medicare patients in the US and 80% have multiple comorbidities, this presents a significant market catalyst for the adoption of connectivity.28
ACCELERATION BY REGULATION
The US FDA has been proactive in improving the regulatory pathway for managing the data generated by software and connected drug delivery devices. The FDA Software Precertification Program, currently in pilot mode, effectively certifies the organisation developing the digital healthcare product rather than looking primarily at the product itself. This approach stems from the nature of software itself, which undergoes rapid, continuous and iterative improvements. This, according to the FDA, will “provide more streamlined and efficient regulatory oversight of software-based medical devices developed by manufacturers who have demonstrated a robust culture of quality and organisational excellence.”29
The most recent development is the FDA’s artificial intelligence (AI) discussion paper.30 Here AI refers to software, which can change its mind and improve its learning or decision making based on new data. While there are already a number of so-called “locked algorithms” approved by the FDA and used in the measurement of stroke risk or detection of diabetic retinopathy, this recent paper aims to tailor to AI’s adaptive nature. Such efforts by the FDA, which aim to simplify and streamline approvals, are expected to accelerate the adoption of connectivity.
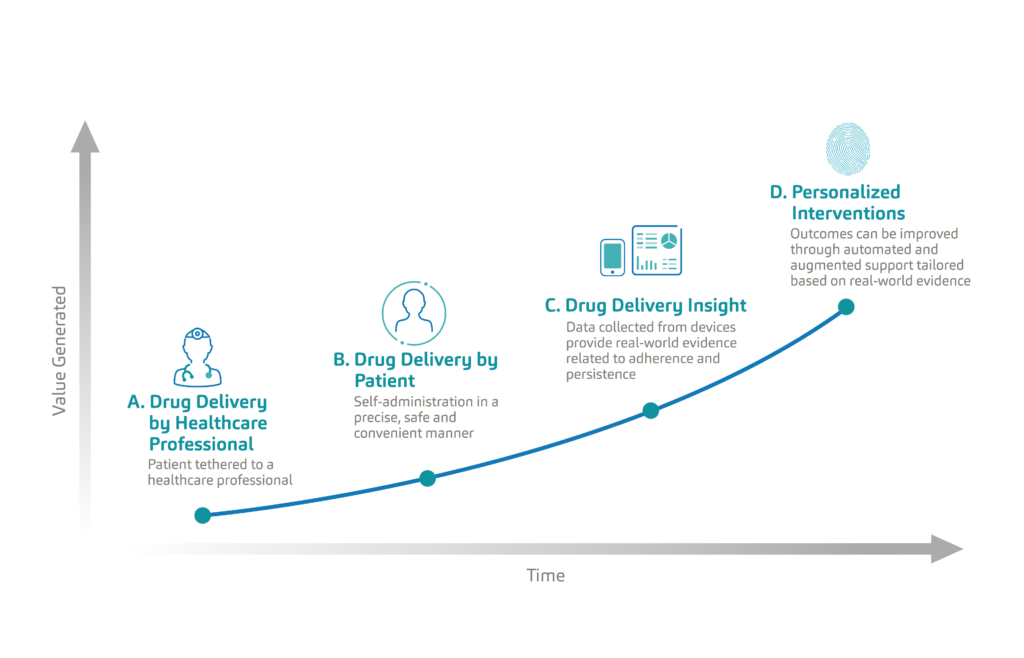
Figure 3: Evolution of the patient experience in drug delivery from clinic-based care to remote personalised care.
CONNECTIVITY TO CARE
When we look at the evolution of the patient experience in drug delivery, we see that patients have traditionally been tethered to healthcare professionals, typically needing to visit a clinic for the administration of their treatment (Figure 3). As autoinjectors set a new standard in drug delivery, patients were empowered to manage their treatments, and do so in a safe and convenient manner. With the adoption of connectivity, drug delivery devices are becoming an important source of real-world data which is now passively measuring adherence. The true value of this data lies in its ability to improve health outcomes through the personalisation of interventions and care delivery. This next frontier in drug delivery, powered by connected therapeutics, will be data driven, personalised, outcomes based and accessible. Only then will connected therapeutics reach their full value.
REFERENCES
- “Novel drug approvals for 2018”. US FDA, 2019.
- “Rapid growth in biopharma: Challenges and opportunities”. McKinsey & Company, 2014.
- “Long-term trends in diabetes”. US Centers for Disease Control & Prevention, 2017.
- “Economic costs of diabetes in the US in 2017”. American Diabetes Association, 2018.
- “FDA Approved Drugs for Endocrinology”. CenterWatch.
- “A1C test”. Mayo Clinic.
- Carls G et al, “Achievement of glycated hemoglobin goals in the US remains unchanged through 2014”. Diabetes Therapy, 2017, Vol 8(4), pp 863-873.
- Kini V, Ho P, “Interventions to improve medication adherence”. JAMA, 2018, Vol 320(23), pp 2461-2473.
- Ivanova J et al, “Impact of medication adherence to disease-modifying drugs on severe relapse, and direct and indirect costs among employees with multiple sclerosisin the US”. J Med Econ, 2012, Vol 15(3), pp 601-609.
- De Vera M, Mailman J, Galo J, “Economics of non-adherence to biologic therapies in rheumatoid arthritis”. Curr Rheumatol Rep, 2014, Vol 16(11), p460.
- Wan J et al, “Inflammatory bowel disease: healthcare costs for patients who are adherent or non-adherent with infliximab therapy”. J Med Econ, 2014, Vol 17(6), pp 384-393.
- Cutler R et al, “Economic impact of medication non-adherence by disease groups: a systematic review”. BMJ Open, 2018, Vol 8(1), e016982.
- Calip S et al, “Medication adherence and persistence over time with self-administered TNF-alpha inhibitors among young adult, middle-aged, and older patients with rheumatologic conditions”. Semin Arthritis Rheum, 2017, Vol 47(2), pp 157-164.
- Abegaz T et al, “Nonadherence to antihypertensive drugs: A systematic review and meta-analysis”. Medicine (Baltimore), 2017, Vol 96(4), e5641.
- Yeaw J et al, “Comparing adherence and persistence across 6 chronic medication classes”. J Manag Care Pharm, 2009, Vol 15(9), pp 728-740.
- Farr A et al, “Retrospective analysis of long-term adherence to and persistence with DPP-4 inhibitors in US adults with type 2 diabetes mellitus”. Adv Ther, 2014, Vol 31(12), pp 1287-1305.
- Shi L et al, “Concordance of adherence measurement using self-reported adherence questionnaires and medication monitoring devices”. Pharmacoeconomics, 2010, Vol 28(12), pp 1097-1107.
- Gallagher B et al, “Are two commonly used self-report questionnaires useful for identifying antihypertensive medication nonadherence?”. J Hypertens, 2015, Vol 33(5), pp 1108-1113.
- “Eroom’s law”. Wikipedia.
- Scannell J et al, “Diagnosing the decline in pharmaceutical R&D efficiency”. Nature Reviews Drug Discovery, 2012, Vol 11, pp 191-200.
- Barrett M et al, “Effect of a mobile health, sensor-driven asthma management platform on asthma control”. Ann Allergy Asthma Immunol, 2017, Vol 119(5), pp 415-421.
- Foster J et al, “Inhaler reminders improve adherence with controller treatment in primary care patients with asthma”. J Allergy Clinical Immunol, 2014, Vol 134(6),pp 1260-1268.
- Chan A et al, “The effect of an electronic monitoring device with audiovisual reminder function on adherence to inhaled corticosteroids and school attendance in children with asthma: a randomised controlled trial”. Lancet: Resp Med, 2015,Vol 3(3), pp 210-219.
- Frias J et al, “Effectiveness of digital medicines to improve clinical outcomes in patients with uncontrolled hypertension andtype 2 diabetes”. J Med Internet Res, 2017, Vol 19(7), e246.
- Yu J et al, “Performance-based risk-sharing arrangements for pharmaceutical products in the United States: A systematic review”. J Manag Care Spec Pharm, 2017,Vol 23(10), pp 1028-1040.
- “Value-based contracts: 2009-Q2 2018”. Pharmaceutical Research & Manufacturers of America, 2018.
- “21st Century Cures Act”. US FDA.
- US Center for Medicare & Medicaid Services.
- “Digital Health Software Precertification (Pre-Cert) Program”. US FDA.
- “Artificial Intelligence and Machine Learning in Software as a Medical Device”. US FDA.

