Citation: “Interview: Mircea Despa and Douglas McClure,” BD. ONdrugDelivery Magazine, Issue 98 (Jun 2019), pp 8-10
Mircea Despa, Associate Director, R&D, leads the Smart Device and Data Sciences group within BD Technologies and Innovation, overseeing digital health R&D, which spans early-stage opportunity assessment to evidence generation through clinical studies. Under his leadership, the group has developed and is currently performing clinical research with BD’s first prototype interconnected disease management system for free-living patients. This system is composed of hardware, software, and data algorithms, designed using behavioural science principles. Prior to this role, Dr Despa led the department’s multidisciplinary efforts to develop smart devices. He has 18 years of experience in the medical, life sciences, and telecommunications industries. Before joining BD,Dr Despa worked at Corning, conducting R&D for optical bio-sensors, biochemical reactors, and specialty optical fibres. He holds a BS in Chemical Engineering from Bucharest Polytechnic Institute (Romania), and an MS in Chemical Engineering and a PhD in Engineering Science, both from Louisiana State University (US).
Doug McClure joined BD in 2016 to help start BD’s Digital Health organisation and capabilities. He has been focused on creating product development and operation processes, and commercial models, for BD’s digital health products and is currently the Platform Leader for the Digital Health in BD Diabetes Care. Mr McClure has been working in the health IT, telemedicine, and digital health industries since 2001. He began his career at Partners HealthCare at Massachusetts General Hospital and Brigham Women’s Hospital (Boston, MA, US) and then worked for a series of companies including a as Co-Founder and Chief Technology Officer of a venture capital-funded startup, Healthrageous. Mr McClure undertook his undergraduate studies at Drew University (NJ, US) and received an MBA from the University of Denver (CO, US).
In this interview, Dr Despa and Mr McClure discuss BD’s approach to connecting devices in its portfolio to meet patient, pharma and other stakeholder requirements. Their discussion focuses in particular on safety and security, highlighting the connected wearable injector, BD Libertas™ with Smart Option, as an example.
Q In the last few years, device manufacturers have either developed connected drug delivery or are currently developing this technology. This effort has exposed many benefits and challenges. Where do you see the market currently, and where is it headed?
“Building connected device technology has become a straightforward engineering task … How you empower
the patient and stakeholder ecosystem with the data,
and how the data is used, have become the more
valuable and commensurately more difficult challenges.”
MD One way to describe the market is to think of the acute care and non-acute care spaces. In the acute care space, it is becoming increasingly clear that there is a growing body of data available for analysis with the goal of driving decision making and improving the outcomes and economics of delivering healthcare.
A large and diverse number of participants are actively working to build and capture value from the data generated in clinical settings. Startups and traditional medtech and technology companies are all in the mix. While some take a go-it-alone approach, most pursue partnerships, which are being formed with the understanding that different expertise and skill sets are best combined in pursuit of common goals.
In the non-acute care space, a significant number of participants are focused on using connected drug delivery solutions for chronic disease management, with diabetes as one of the leading areas of participation and perceived opportunity. There is an increased expectation in the level of activity in this space as more evidence is generated showing that connected solutions generate value for a number of stakeholders, including the patient, providers, pharma, and payors.
DM Building connected device technology has become a straightforward engineering task. Building a system that supports and leverages the connected device data is more complex but manageable for organisations with the right capabilities. What these solutions have highlighted is that connecting the data is just the first step. How you empower the patient and stakeholder ecosystem with the data, and how the data is used, have become the more valuable and commensurately more difficult challenges.
As always, the market will reward those devices and systems that best address the unmet needs. In the context of connected devices this is not just about transmitting the data from the device. What we see in the industry is a move to address those unmet needs and expectations of the user once the data is in their control. When we meet those needs we drive engagement, and when we drive engagement we drive adherence – this is when we truly unlock the value of connected device solutions.
“Technology developers are in a constant pursuit to
resolve risks and improve performance and profitability; in general, the technical hurdles are more obvious and seemingly easier to focus on. In contrast, stakeholder adoption is lagging, likely as a result of a combination of reasons. For example, only now are we beginning to see emerging sets of data demonstrating value.”
Q The connected drug delivery device market is growing quickly and so too is the Internet of Medical Things (IoMT). What impact is this having on stakeholder adoption, technology and infrastructure?
MD IoMT is a term that is being increasingly used to describe a wide range of existing and potential solutions in the hospital, outpatient, home, or other clinical spaces. Increasingly, the participants in this space express optimism that interconnected devices bring with them the opportunity to collect and combine data in order to generate new value. However, there is a clear dichotomy between the advent of technologies and supporting infrastructure and stakeholder adoption. Technology developers are in a constant pursuit to resolve risks and improve performance and profitability; in general, the technical hurdles are more obvious and seemingly easier to focus on. In contrast, stakeholder adoption is lagging, likely as a result of a combination of reasons. For example, only now are we beginning to see emerging sets of data demonstrating value. The known slow pace of adopting novel technologies by a typically conservative industry is also a factor, of course.
DM The lines between consumer devices are blurring in the consumer’s mind. Today’s consumerexpects connected experiences for all of the products and services they invite into their lives. The Internet of Things (IoT) in everyday use is driving expectations for what IoMT must be able to accomplish and this certainly calls for device data to be available to access, use and share as needed. But connected experiences go beyond just device data. Patients will expect connected experiences that empower them to self-support, self-manage and reach on-demand support – anytime and anywhere.
This means that when we are building IoMT products we need to think about the entire stakeholder experience. When the patient needs help with their connected medical device, they want to know how they can they leverage those same connected device technologies in the same way and with ease as the other technology tools they are using today.
IoMT devices also carry the challenge that expectations go beyond those IoT devices. IoMT must meet the expectations that people put on medical and health technology. The infrastructure we deploy for these patient-centric IoMT solutions has to be ready to meet those needs of the entire user experience – anywhere, anytime, securely, and most importantly always ensuring safety.
“The lines between consumer devices are blurring in the consumer’s mind. Today’s consumer expects connected experiences for all of the products and services they invite into their lives. The IoT in everyday use is driving expectations for what IoMT must be able to accomplish.”
Q BD has long been a proponent of using connected technology in healthcare. How has BD’s approach and philosophy regarding connected technology evolved?
DM In the last couple of years BD has become more active in connected solution development. BD’s businesses that have a much more direct to consumer interaction have led the way in developing an enterprise connected health infrastructure. Today the platform supports the patient experience of our connected technologies with a focus on patient engagement, adherence, and improved outcomes. This platform also supports the use of ecosystem connected devices that are companions to our unconnected devices. These BD businesses are preparing global launches of their connected devices that will leverage the capabilities built. The best way we know that this set of capabilities is ready to be used by our customers is to use it at scale ourselves.
MD BD has advanced itsapproach in this space by bringing into the marketplace a first mobile app solution for diabetes support and implicitly developing a robust infrastructure that supports safe and secure data transmission and storage, under the strict HIPAA [US Health Insurance Portability and Accountability Act 1996] and GDPR [EU General Data Protection Regulation]. Additionally, we leveraged this infrastructure and learnings when developing the app for the BD Libertas™ with Smart Option app (Figure 1).
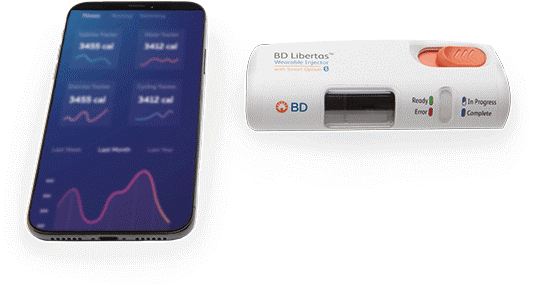
Figure 1: BD Libertas™ wearable injector was designed from the outset with the capacity for smart features, simply by adding a smart element to the core device.
Q Drug delivery devices typically incorporate connectivity in one of two ways: through integration or add-on capability. Please describe for us BD’s approach and how might this approach be beneficial to pharmaceutical companies seeking connected devices?
MD At BD, we have pursued both paths to align with the business units’ existing portfolios and roadmap strategies. Early on we recognised that add-ons come with the advantages of not impacting the function of the already-marketed base devices and the possibility to amortise the associated costs of add-ons over multiple use events. Unfortunately, add-ons come with the misperceptions of impacting workflow and complex technology requirements needed to add sensors and electronics without impacting device function.
Alternatively, integrated designs alleviate technology complexities while improving performance and reducing cost of the solution. Integrated devices do however require drug-device combination testing, evidence generation, and associated regulatory filings.
“The best way to manage all of these risks effectively is to approach them holistically and with a systems view. Security and privacy are not just cloud hosting concerns. Usability is not just a physical device concern. Cost is always best managed at scale.”
DM I completely agree with Mircea. I see our approach being more focused on integration and less so as an add-on. If you consider connectivity early in your device development it helps you to design the proper system architecture around it and this enables a more seamless integration with existing and future infrastructures and devices. For pharmaceutical companies this will mean that BD devices will be compatible with a wider variety of infrastructures, be compatible with each other and will operate with a high reliability, acceptanceand use.
Q There are many risks when considering connected drug delivery devices such as cost, infrastructure, usability, and data privacy to name a few. How does BD mitigate some of these risks with the BD Libertas™ wearable injector with smart option?
DM When building medical technology that will be used in the field by patients and stakeholders the risks can feel overwhelming. The best way to manage all of these risks effectively is to approach them holistically and with a systems view. Security and privacy are not just cloud hosting concerns. Usability is not just a physical device concern.
The BD Libertas™ with smart option is built upon BD’s Enterprise Digital health platform. This medical grade platform features a highly scalable and secure cloud infrastructure, cross platform mobile client capability, and mobile wireless radios connectivity. Leveraging this set of platform capabilities, we address entire experience usability while ensuring security and privacy across the entire customer interaction.
MD We have applied a robust approach to innovation bringing life to systems that deliver on unmet needs and providing functionality that truly makes a difference. To arrive at this result, we carefully examined and understood what matters most for patients, pharma and other stakeholders that can benefit from the smart features and associated data.
The primary goal was to design and develop a solution that is both safe and secure and built on enterprise architecture that was developed by many experienced hardware and software engineers. We used an agile approach to product development, constantly iterating to deliver the highest value functionality at the lowest cost. Finally, we designed safety and security into the system and tested to ensure we delivered on all our goals. We are proud that we have accomplished that.

