Citation: Wiglenda M, “A Five-Step Approach to Innovation in Drug Delivery Systems. ONdrugDelivery Magazine, Issue 99 (Aug 2019), pp 47-52.
Michael Wiglenda explains Gerresheimer’s industrialisation offering and the five-step Gate-Model that guides its innovation process.
“The aim is to develop new and better products that have a higher chance of succeeding in the competitive marketplace.”
Gerresheimer has a long track record of helping clients navigate the hurdles that stand in the way of transforming a drug delivery system concept into a mass-produced product ready for market. Less well known is the fact that, behind the scenes, one of the secrets of its success is the way it navigates using its own Gate-Model to guide its innovation process.
The Gate-Model is a five-step process covering the crucial stages of product development – from the concept phase through design and development and pre-production development to mass production development and, eventually, mass production. The aim is to develop new and better products that have a higher chance of succeeding in the competitive marketplace.

Figure 1: The small batch production facility has a room with a vacuum for filling toxic materials and, depending on the requirement, it may be done in a protective atmosphere.
The first stage of the Gate-Model – the concept phase – involves developing ideas and product concepts for a client’s new device. Using an understanding of marketrequirements, the industrial design is drafted, with Gerresheimer taking care of patent management too. Operational concepts are developed as part of the usability engineering, with critical sub-functions considered and models created. In addition, Gerresheimer analyses design concepts as part of usability studies – and performs concept evaluations as well as feasibility studies. The result of the concept phase is a preferred concept, along with a thorough risk analysis and considered market requirements.
Next comes the design and development phase when product requirements are defined, the design is broken down and a client’s product is developed in accordance with the relevant regulations. The process required to manufacture the product is developed – and analysed for potential patenting – and simulations and tests are conducted. Functional samples are also created at this stage.
The Gerresheimer service package also includes preselection of suppliers, selection of materials and design of the packaging. The result of this second stage of the Gate-Model is a completely developed product with defined production processes and a design freeze.
The third phase of the process covers pre-production development, which includes design verification and preparation for product verification. Moulds are developed for small batch production, along with specialised machines, and measuring equipment is developed in Gerresheimer’s quality laboratory. If necessary, development samples, clinical samples or stability batches are manufactured in a small batch production facility (Figure 1) complete with a clean room (Figure 2) – which can also accommodate low-volume commercial production. The result of this phase is a verified product.
Phase four of the Gate-Model is mass production development – involving industrialisation, validation of production means and preparations for introduction of the product. Mould-making and automation engineering experts design, develop and build the high-cavity injection moulds used for mass production (Figure 3), along with any complex robots and systems required. Gerresheimer also qualifies the production equipment, validates the processes and qualifies suppliers – and prepares the product master file. The result of this stage is mass production means and a product validated for mass production.
The fifth and final phase of the process is mass production, when the product is introduced and lifecycle management begins. The result of this phase is ongoing production of the standard parts and/or standard products.
FULL SERVICE PROVIDER
The Gate-Model illustrates how Gerresheimer is a full service provider of drug delivery systems, operating as a one-stop shop for its clients. It has experience across a variety of delivery routes – including inhalers, pen-injectors, autoinjectors and prefilled syringes – administering active substances via pulmonary inhalation, through the skin or via mucous membranes. It works with clients to develop and manufacture both primary and secondary packaging for diverse drug products – ensuring they are convenient, patient-friendly and delivered to where they are needed quickly and efficiently.
Clients starting a project with Gerresheimer will find a plethora of flexible options available, whether they are looking to develop an existing project or begin from an initial idea. However, Gerresheimer is also a partner for industrialisation of completed drug delivery device projects – working with finalised designs and taking them through to mass production. Projects and products looking to beoptimised for polymer process are managed by Gerresheimer’s Technical Competence Centers (TCCs) in Wackersdorf and Bünde (Germany), Peachtree City (GA, US) and Dongguan City (China).

Figure 2: Gerresheimer’s small batch production facility has a class 8 clean room in accordance with DIN EN ISO 14644-1.
TECHNICAL COMPETENCE CENTERS
The TCCs are the “technical heart” of Gerresheimer Medical Systems, in terms of both products and processes. Using the simultaneous engineering method, a TCC maps the entire development process of a product all the way from where it picks up the project through to full-scale industrial production. In the TCCs, designers, engineers and technicians work hand in hand, resulting in drug delivery systems characterised by high quality, functional reliability and capacity to be mass produced in a way specifically designed for plastics.
For example, TCC engineers ensure that all the individual parts of an inhaler can be easily assembled into a solid product to ensure optimum functionality. This care and attention extends to pre-production, applying the highest standards to material selection and assembly technique, as well as drug delivery system functionality.
The TCCs’ capabilities include:
- Small batch production with an ISO 14644-1 Class 8 cleanroom
- Pilot plant
- Qualification and validation for moulds and special-purpose machines
- Quality laboratory, including its own measuring room with product-specific test equipment
- Functional testing lab
- Mould making and optimisation
- Special-purpose machinery manufacture.
As well as providing design and engineering expertise for polymer components, the TCCs are able to handle production during development, after which full-scale production takes place at Gerresheimer’s international production facilities in North and South America, Asia and Europe.
“The integrated approach from Gerresheimer has a number of advantages for clients, including reduced development time and
risks – meaning products get to market faster.”
This integrated approach from Gerresheimer has a number of advantages for clients, including reduced development time and risks – meaning products get to market faster.
MOULD MAKING
The mould-making department at Gerresheimer Medical Systems has been manufacturing sophisticated injection moulds since 1958. Its precision injection moulding tools are designed to meet the requirements of the pharma industry relating to precision and size accuracy, surface quality and high output quantities. They are characterised by 100% repeat accuracy, durability and optimised temperature control for short cycle times.
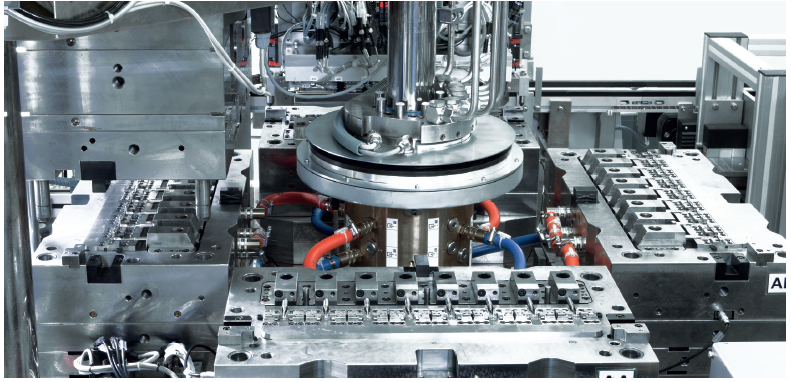
Figure 3: Rotary table with four bottom tool halves for insert moulding of a cannula with ABS for an infusion set.
For drug delivery systems, Gerresheimer uses moulds with needle valve nozzles to avoid the formation of strands – i.e. there is no particle formation during separation and removal of the sprue from the mould. It also builds moulds out of rust-proof steel for use in a clean-room environment and ensures there is good ventilation for the moulded parts in the cavities, preventing burn-up and the collection of deposits. Production is fat-free due to the smooth coating of all moveable parts, as well as clean and material-appropriate part removal, achieved via inclined removal surfaces that avoid abrasion.
“Uncompromising quality assurance has the highest priority across the entire production process.”
The mould-making department represents an efficient method of operation, ensuring fast and smooth production of moulds by a segmented structure in mouldproduction and modification, examining potential changes with test moulds. Furthermore, the department works with replaceable mould inserts for short maintenance and repair times without the need for additional adaptations. Data consistency from the design to all machines and workbenches, as well as the direct link to quality assurance (QA), ensures that moulds are of the highest quality.
Quality Assurance
Uncompromising QA has the highest priority across the entire production process. Precision moulds are ultimately the prerequisite for excellent product quality. As such, only the latest measuring equipment and techniques – for example, computer numerical control (CNC) image processing – are used in the internal measurement lab.
Modern Mould Technologies
More than 65 specially trained employees produce:
- Low- and high-cavity injection moulds, up to 128 cavities, with precision in the micrometre range
- Single- and multi-component moulds
- Indexing plate moulds
- Hot-runner injection moulds
- Moulds for insert moulding – needle and lancet encapsulation
- Stack moulds.
Award-Winning Mould-Making Department
The quality of Gerresheimer’s mould-making department was recognised by its top placement in the renowned Excellence in Production competition, organised by the Laboratory for Machine Tools (RWTH Aachen University, Germany) and the Fraunhofer Institute for Production Technology (Aachen, Germany). The TCC of Gerresheimer Regensburg GmbH was named Mould Maker of the Year 2014.
PILOT PLANT
“One focus of expansion is the establishment of small batch production for prefillable glass syringes and cartridges.”
The TCC pilot plant is the practice-oriented competence centre for all injection moulding processes. Here, Gerresheimer proves moulds to check performance and measures, optimises and qualifies moulds. Moulds are sampled using special machinery under near-series conditions and subjected to comprehensive application and processing tests to get them ready for large-scale production.
The sampling and mould optimisation process in the pilot plant forms the basis of the entire component verification. An important stage during this process is the set-up of stable parameter settings for injection moulding, based on a fractional factorial design of experiments (DoE). Additionally, it is at this point that the optical and dimensional component measurements take place in the certified measuring lab, which are then documented in a comprehensive sample test report. Machine and process-capability documentation and mould trials over set time lengths (e.g. four or 24 hours) complete the pilot plant phase.
ANALYSIS AND TESTING
Quality Laboratory
When it comes to drug delivery systems, safety is of the utmost priority. The pilot plant therefore carries out extensive testing in the areas of materials, geometry and function. Gerresheimer has a measuring lab for the geometric measurement of components, assembly units and finished products, a lab for material analyses and a lab for functional testing with product-specific testing equipment.
Optical & Tactile Measurement Technology and Industrial Computer Tomography
By using a measurement lab with the most modern measuring equipment, Gerresheimer ensures that complex mould inserts and filigreed injection moulding parts or assembly units can be measured to extreme levels of precision. The complete set of component measurements is documented in an initial sample test report.
The measurement equipment includes:
- Various multi-sensor co-ordinate measuring machines for optical and tactile component measurements
- Universal co-ordinate reading microscopes
- An industrial computer tomograph for the destruction-free measuring and testing of individual drug delivery components or entire assemblies.
Material-Specific, Physical and Chemical Analyses
The material analysis lab is responsible for the inspection and approval of incoming goods and raw materials worldwide. The standard spectroscopic and thermal analyses are:
- Fourier-transform infrared spectroscopy (FTIR)
- Melt mass-flow rate (MFR)/melt volume-flow rate (MVR)
- Differential scanning calorimetry (DSC)
- Thermogravimetric analysis (TGA).
In addition to these, Gerresheimer’s extensively equipped lab also offers the possibility of a physical-chemical analysis of viscosity, residual moisture and density, as well as an infrared spectrometer and a thin section microscope. In-house expertise enabling development and execution of customer-specific methods rounds off Gerresheimer’s analysis portfolio.
Product-Specific Functional Testing
In the functional testing lab, Gerresheimer develops and qualifies test methods to guarantee compliance with product-specific requirements. It ensures enhanced safety for patients via comprehensive testing of physical product characteristics, product-specific performance tests and statistical data analysis during the product development cycle.
Individual Qualification Packages The pharmaceutical and medical product industry requires proof of process capability and the reproducible production of an injection mould. QA is therefore given critical importance in both national and international laws and guidelines, signifying a requirement for increased effort and expense with respect to the qualification and validation of moulds in the development and industrialisation phases. As a result of these regulations however, there is less wear on moulds and a higher quality of parts overall, resulting in less waste. But mould qualifications are time and cost intensive. This is why Gerresheimer offers its customers variousmould qualification levels depending on the product, its area of application and the regulatory requirement level.
AUTOMATION ENGINEERING
Together with the development and construction of special-purpose machines associated with moulds, Gerresheimer Medical Systems offers its customers high-performance automation solutions. In the pharma and healthcare industries, automation co-ordinated precisely with the product, project and processes has a decisive influence on the quality and economic efficiency of production. The technicians, mechanics, electricians, designers, qualification experts and programmers from the automation engineering department are responsible for this task at the TCCs.
The automation engineering department provides automation competency; develops automation solutions; and specifies, designs, builds, procures and qualifies the following:
- Customer- and part-specific assembly lines
- Testing robots (pressure, flow rate, optical features, force deflection systems)
- Rotary table systems
- Linear systems
- Robots to insert and remove parts
- Packaging systems
- Pre-production equipment
- Pharmaceutical assembly systems
- New generations of glass forming lines (Figure 4).
All the production systems produced by Gerresheimer meet good automated manufacturing practice (GAMP) requirements, as well as US FDA 21 CFR Part 11, and are designed for production in clean rooms in accordance with ISO 14644-1 Class 7/8 or GMP Grade C/D. Being an international manufacturer, Gerresheimer also monitors and assists the start-up of its production equipment on the client’s site.
The assembly steps and inspection of the modules are done by intelligent camera and inspection systems. As an example, a respiratory patient must be able to easily determine how many doses remain in their inhaler – to avoid the risk of accidentally running out of medication. To facilitate this, Gerresheimer designed an assembly system where the dose-counter function is checked both with a camera inspection system (camera control of the tab position) and a position sensor in the display element after a simulated number of doses, all of which was fully automated.
Automation is an integral component of Gerresheimer’s product and process development, and leverages its expertise and know-how throughout the concept and design phase. This means Gerresheimer doesn’t wait until mass production to develop automation solutions – it develops them in the prototype and pre-production phase, saving a lot of time.
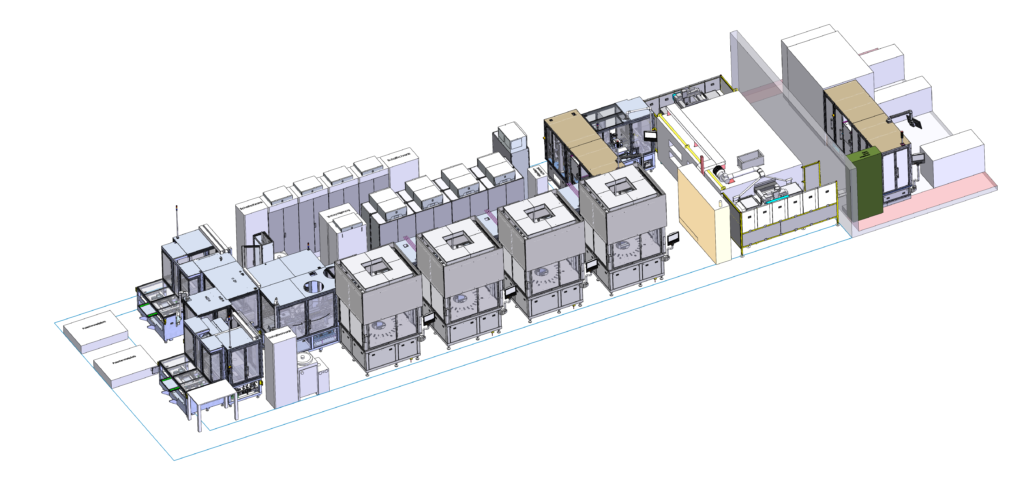
Figure 4: Design, development and build of new generations of glass forming lines as well as customer- and part-specific automation solutions.
SMALL BATCH PRODUCTION
Prior to series production, pharmaceutical and medical technology products run through an exhaustive approval process, for which small numbers of units need to be produced repeatedly. For example, small batches may be required as clinical samples, development samples or stability batches.
With its TCCs, Gerresheimer offers clients its own production systems for this task, which are capable of quick and uncomplicated production of development samples, clinical samples or small series at any point in the project.
These facilities include the Class 8 clean room and some of the injection moulding machines have integrated laminar flow covers to create a Class 7 clean-room environment in the injection-moulding area. Project-specific assembly units and specific measuring technologies complete the equipment.
Expansion into Glass
Gerresheimer has expanded its Wackersdorf TCC. The company has invested tens of millions of Euros in creating 3,000 m2 of additional space for the development and industrialisation of glass products, such as syringes and cartridges. The task area of the TCC has thus been expanded to include working with glass. Construction began in 2018.
One focus of the expansion is the establishment of small batch production for prefillable glass syringes and cartridges. Once the project is complete, it will be possible to produce pre-series modules from glass forming to ready-to-ship, washed and siliconised ready-to-fill systems. The focus is on syringes and cartridges for sophisticated, biotechnologically manufactured medication, clinical samples for approval or prototypes for process and technology development.
At the same time, glass competence is also being established in the automation systems area (special machine engineering) to develop innovative technologies for glassforming and automation. In the future, new generations of glass forming lines for syringe production will originate in a co-operation between Gerresheimer’s Bünde and Wackersdorf locations, with small batch production and automation systems in Wackersdorf and large batch production in Bünde. This expansion is set to greatly improve Gerresheimer’s capacity to develop, optimise and produce innovative glass-based drug delivery systems.
CONCLUSION
Using the equipment and latest technologies in its TCCs, Gerresheimer handles:
- Concept development
- Concept studies
- Ratings and cost analysis
- Industrial design
- Product development
- Process and manufacturing equipment design
- Mould making
- Production (clinical sample, small batch, large batch)
- Automation engineering
- Product Assembly (manual, semi-automated, automated)
- Product finishing
- Pharmaceutical assembly and filling
- Sterilisation
- Packaging
- International distribution.
Gerresheimer is able to join at any phase of drug delivery system development – from initial concept through to final design – and optimise the product for mass production with specialist knowledge and high-quality processes and facilities.

