Citation: Guillard B, Farjas P, Chandra A, “Patient Safety: Key Driver to Preservative-Free Nasal Spray Development”. ONdrugDelivery Magazine, Issue 102 (Nov 2019), pp 18-21.
Benoît Guillard, Pascale Farjas and Audrey Chandra look at the effects of preservatives in nasal sprays, and the challenges and advantages of delivering preservative-free nasal spray drugs.
Current challenges in treating patients who suffer from chronic diseases have encouraged pharmaceutical companies to continue innovating to tackle the problems encountered by patients. The quality of life of these patients depends highly on their clinical outcomes, which are based on their adherence to the prescribed treatment as well as on the efficacy of their treatment.
The adverse effects of treatment may affect patient compliance. For example, the use of preservatives in a nasal spray may affect patient adherence due to its risks and possible side effects. Innovation in nasal delivery therefore becomes a crucial need for patient safety. By using a preservative-free nasal spray on a daily basis, patients should worry less about the adverse effects of their treatment – which therefore encourages better patient compliance.
THE EFFECTS OF PRESERVATIVES IN NASAL SPRAY
“Different types of preservative found in nasal sprays may have different undesirable side effects on the physiology of the nose cavity.”
If we take a closer look at nasal drug products delivered through a device for chronic diseases, it mainly concerns locally acting drugs for allergic patients – i.e. nasal sprays. The nasal mucosa plays an important role in mediating immune responses to allergens and infectious particles which enter the nose. It helps prevent allergens and infections from invading the nasal cavity and spreading to other body structures – for example, the lungs.
The nose cavity is lined with a type of epithelium where cells arrange themselves in columns and project tiny hairs called cilia which contain mucus-producing cells (goblet cells). Different types of preservative found in nasal sprays may have different undesirable side effects on the physiology of the nose cavity, which are observed in various studies.
However, the use of preservatives in nasal sprays has become controversial. Some argue that a certain amount is well tolerated by patients, whereas others state that they might increase the risk of adverse events for patients. For instance, an in vitro study shows that benzalkonium chloride (BAC) can cause ciliotoxicity (impaired ciliary activity) of the nasal mucosa – and therefore nasal irritation.1
In a wide range of clinical trials, which used mostly nasal sprays containing BAC, various adverse effects have been observed. These side effects include increased mucosal swelling and nasal hyper-reactivity, type IV hypersensitivity, decrease of mucociliary clearance, and nasal mucosa dysplasia. On the other hand, some clinical studies claim no effect on ciliostatic and no toxic effect.
Although the use of preservatives in nasal sprays is debatable, risks havebeen reported by different studies and, consequently, the European Medicine Agency (EMA) recommends zero BAC in nasal sprays. In fact, the German Federal Institute for Drugs and Medicinal Devices (BfArm) has proposed that BAC is removed from intranasal products in Germany because of concerns about mucociliary effects.2 Keeping in step with the regulatory trend, and applying the precautionary principle for patients, Nemera has developed preservative free approaches.
KEY BENEFITS OF ADVANCIA® PF WITH PUREFLOW TECHNOLOGY
“Recent studies have demonstrated that bacterial
transfer through the membrane structure takes place during filtration operations, even if the pore size is significantly smaller than the bacteria size.”
Driven by the regulatory recommendations for preservative-free formulations in nasal sprays, as well as the desire to protect patients from adverse events, a technology breakthrough is a fundamental requirement when designing a preservative-free drug delivery device. Instead of using preservatives, an alternative way of keeping a nasal spray sterile is by preventing bacteria entering and contaminating the drug formulation.
Nasal sprays can be contaminated through their drug delivery orifices, with bacterial contamination coming from the external environment or the patient. A mechanical closing tip ensures that no contamination can be introduced in the nasal spray orifice after the spray has been dispensed.
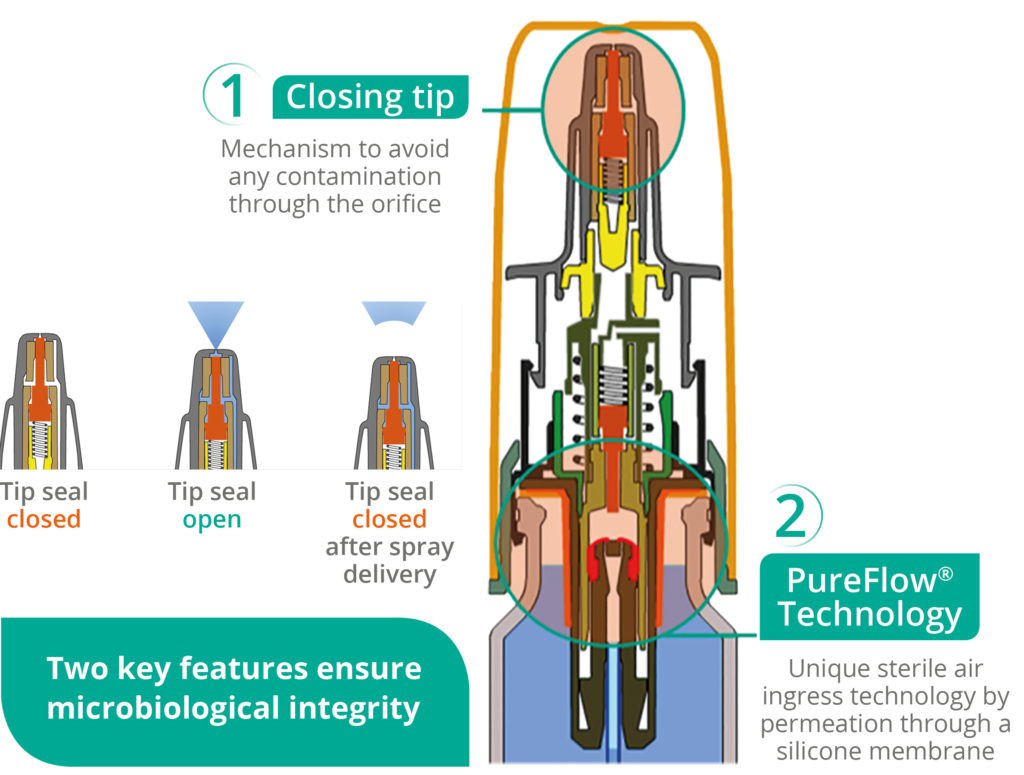
Figure 1: Advancia® PF illustration.
To prevent contamination via air entering the device, most commercialised nasal sprays use a filter system which stops the entry of bacteria into the container. Airborne bacteria are typically around 0.3 μm. However, other smaller bacteria are present.3 Furthermore, recent studies have demonstrated that bacterial transfer through the membrane structure takes place during filtration operations, even if the pore size is significantly smaller than the bacteria size.4
Nemera has introduced an alternative to filters for the Advancia® PF (Figure 1) nasal spray – using a silicone membrane to filter the returning air. The intake of air into the dispenser takes place via a venting system with a silicone membrane called PureFlow technology (Figure 2). This patented technology has a continuous barrier of homogeneous material which allows air to diffuse through the silicone, which acts as a permeable membrane. Consequently, the continuous barrier guarantees the microbial integrity of the drug.
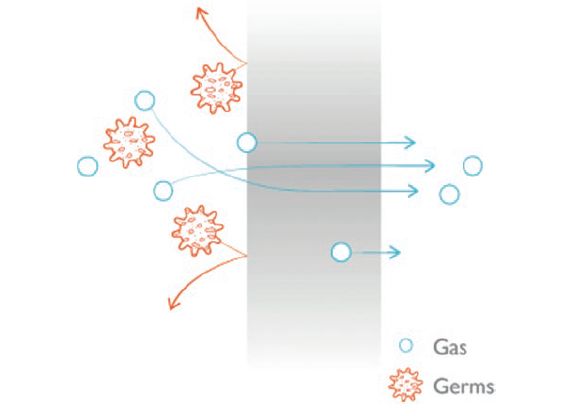
Figure 2: PureFlow technology.
The venting system filters the intake of air using a very fine membrane manufactured from silicone polymer. The silicone membrane is a solid, non-porous material. As it is homogeneous and does not contain any holes, it can be precisely engineered. The membrane’s intermolecular distance is of the order of nanometres – allowing the passage of air through the membrane but completely preventing the passage of any liquid or solid particle, including bacteria, due to the silicone membrane structure where the size is smaller than 0.3 μm.
The function of the silicone membrane can be compared to an inflated balloon. The balloon is a continuous, waterproof material yet gas slowly passes through the wall of the balloon until the pressures inside and outside reach equilibrium. Devices that use this technology can be tested individually in-line as a consistent part of the manufacturing process to ensure robust quality standards. This provides an even greater assurance of safety for patients.
Moreover, Advancia® PF offers a patented anti-clogging actuator in the upper part of the system, called closing tip. This mechanism ensures that no contamination can enter through the actuator orifice, which therefore provides protection from crystallisation and clogging issues, and avoids evaporation to guarantee good prime retention.
PERFORMANCE EVALUATIONOF ADVANCIA® PF
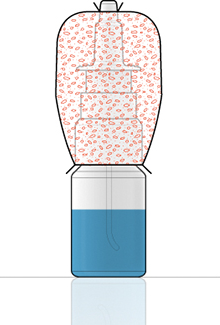
Figure 3: Closure venting integrity test.
To assess the performance of Advancia® PF, the microbial integrity has to be evaluated by two common practice methods.5,6 The first standard contamination test is done by immersing the applicator inside a contaminated petri dish. The second test uses a contaminated air suspension which contains bacteria. These tests are used to verify the performance of the nasal spray; to prove whether or not the formulation inside the nasal spray is contaminated after repeated spray actuations.
The closure venting integrity test (Figure 3) is done by using Bacillus subtilis with a concentration between 108 and 109 CFU/g on an anti-static spherical aerosol size of a few μm. The test protocol requires nutritious sterile solution and the test has to be done on 20 samples.
Firstly, the pump has to be assembled on a bottle. Then, the sterility of the assembly must be checked after the assembly of the device, before exposure to the bacteria, with an incubation conducted for several days at more than 30˚C. Finally, an elastomeric sleeve has to be put around the pump and vial, and the gap between the pump and the vial is filled with the contaminated powder. The sleeve is sealed to the pump and vial. The pumps are actuated a number of times and the sterility of the nutritious solution is checked, with incubation for several days at more than 30°C. The test will therefore show whether the solution is contaminated or not.
The second contamination test is called a tip seal integrity test (Figure 4) and uses Pseudomonas aerugiosa with a concentration of 107 CFU/ml in a sterile peptone water solution inside a petri dish. The test is also done on 20 samples. The pump is assembled on a bottle filled with peptone water and incubated for several days at more than 30˚C to check the microbial contamination of the device following assembly and before exposure to the bacteria. Over the course of eight days, the pumps are actuated a number of times – with the orifice tip of the nasal spray immersed in a contaminated solution – and some sprays are collected in a petri dish to evaluate contamination of the spray. This test is done to check if the spray has any microbial load.
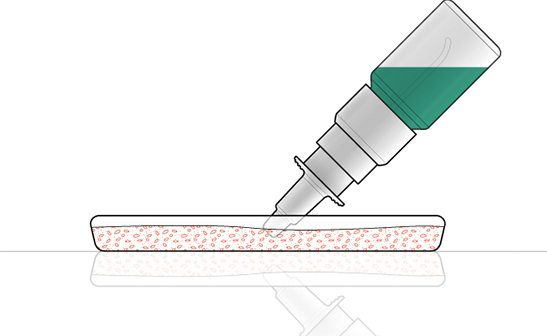
Figure 4: Tip seal integrity test.
All 20 pumps of Advancia® PF with PureFlow technology passed both the tests – the closure venting integrity test and the tip seal integrity test – since no bacterial ingress in the container or on the tip was observed.
ENSURING PATIENT SAFETYTHROUGH ADVANCIA® PF
Patients should be the key driver for innovation breakthroughs as patient safety should be the number one priority. Understanding what are the pain points for the patient is an important element in nasal spray development. Implementing different precautions for patient safety is crucial.
In the case of patients using nasal sprays daily over several weeks – for instance, to relieve allergy symptoms – it makes sense to keep preservatives away from the formulation to avoid adverse effects such as nose irritation. Applying this precautionary principle for patients, Nemera has therefore developed Advancia® PF to deliver preservative-free nasal spray drugs, as well as to keep up with the regulatory trend.
REFERENCES
- Riechelmann H et al, “Nasal toxicity of benzalkonium chloride”. Am J Rhinol, 2004, Vol 18(5), pp 291–299.
- Elder P, Crowley P, “Antimicrobial Preservatives Part Three: Challenges Facing Preservative Systems”. Am Pharm Rev, Jan 1st, 2012.
- Salem H, Katz S, “Aerobiology: The Toxicology of Airborne Pathogens and Toxins”. Book, Published by Royal Soc Chem, April 2016.
- Wang Y et al, “Influence of size, shape, and flexibility on bacterial passage through micropore membrane filters”. Environ Sci Technol, 2008, Vol 42, pp 6749–6754.
- Bommer R, Kern J, Hennes K,Zwisler W, “Preservative-free nasal drug-delivery systems”. Med Device Technol, 2004, Vol 15(9), pp 14–6, 18.
- Bagel S, Wiedemann B, “Extension of in-use stability of preservative-free nasalia”. Eur J Pharmaceutics Biopharmaceutics, 2004, Vol 57(2), pp 353–358.

