Citation: Trgovac S, Ford R, “How Digital Technology Can Transform Clinical Trials. ONdrugDelivery, Issue 113 (Oct 2020), pp 42-45.
Sheila Trgovac and Rebecca Ford suggest that now is the time for innovation in clinical trials – using a patient-centric approach whilst leveraging digital solutions to create a digital clinical trial ecosystem.
The healthcare industry continues its rapid growth year over year, with pharmaceutical development leading the pack. In this highly regulated industry, the regulatory agencies put patient safety ahead of all else – requiring rigorous, highly controlled clinical trials as part of the development process.
“Ensuring patient adherence to clinical protocols in an at-home setting is not a trivial problem to solve.”
Clinical trials are incredibly costly and time-consuming endeavours. The average cost to conduct a Phase III trial is estimated at US$20 million (£15.5 million), with a median of $41,117 (£31,876) per patient and $3,562 (£2,761) per patient visit. These expenses have reportedly risen by 100% in the last 11 years.1 A significant contributing factor in these remarkable costs – and why clinical trials often falter – comes down to reliable patient engagement. Today, less than 5% of the US population participates in clinical research,2 with 86% of trials not meeting their enrolment timelines due to issues with recruitment.3 Patient dropout is also a common problem, with 85% of all clinical trials failing to retain enough subjects to successfully complete a study.4 These statistics are not surprising, as ~80% of potential participants are living more than two hours away from the nearest clinical trial site.2
With a push to lower the commercial price tags of new drugs – and find ways to get them to market sooner – pharmaceutical companies and regulatory bodies are increasingly more open to new clinical trial methodologies and tools, including self-administration at-home dosing, more patient-centric study designs, remote data collection and telehealth touchpoints. Innovative technologies – such as inexpensive sensors, embedded wearables, GPS, more ubiquitous WiFi connections and cloud-based data integration – are enabling easy, real-time, automated data capture.
In parallel, during the current covid-19 pandemic, the pharmaceutical industry is further forced to shift away from traditional clinical trial modalities with a bricks-and-mortar approach – where patients must go to a clinical site for dosing and follow-up – to a more patient-centric approach where the trial comes to the patients in the form of digital enablement. In a period of just a few months, 1,100 clinical trials were disrupted due to lockdown mandates, limited access to clinical sites and people’s shift in priorities and comfort levels.5
The US FDA encouraged new strategies when it issued new guidelines in March 2020 for clinical studies that included “evaluating alternative methods for assessments, like phone contacts or virtual visits, and offering additional safety monitoring for those trial participants who may no longer have access to the investigational product or the investigational site”.
CLINICAL TRIAL OPPORTUNITIES
“Through innovative digital technologies, system solutions can be created and integrated to support the full clinical trial ecosystem.”
Now is the time for innovations in clinical trials to provide a patient-centric approach to driving patient engagement and capturing remote and accurate clinical data (including primary endpoints and patient-reported outcomes) and to drive down clinical trial costs. Imagine a clinical trial that is routed in design around patient comfort yet enables the data capture needed to successfully ensure the safety and efficacy of a novel drug substance. Ximedica is uniquely suited to create and execute such a solution by leveraging technology and data analytics to remotely engage and monitor patients.
DRIVING ADHERENCE
Ensuring patient adherence to clinical protocols in an at-home setting is not a trivial problem to solve. Ultimately, patient adherence to the clinical protocols – including dosing and follow-ups – drives the data collection needed to successfully meet the safety and efficacy parameters laid out for successfully taking the new drug to market. Developing product systems combining thoughtfully designed hardware with digital solutions can get us to a place of adherence. Considering both designing for the user and the use environment enables patients to stay on track with minimal disruption to their daily lives.
Simple considerations as to when and where the patient is administering the drug provide significant insight into the needs for clinical trial. For example, should the system fit next to the patient bed or on a side table? Do the patient monitoring solutions need to be worn all day? Does the drug product require specific storage conditions? How often is the drug administered – daily, weekly, monthly? In the case of a longer latency between dosing or clinical follow-ups, using adaptive artificial intelligence (AI) can drive adherence through simple reminders to an app on your phone or an alarm linked to the data capture or packaging components of the drug delivery device. All of these considerations allow for more thoughtful solutions that ultimately aid, and potentially motivate, the patient to stay on track with the clinical trial.
Another consideration when it comes to keeping patients engaged whilst leveraging the current digitally driven times involves the deployment of multi-channel communication to provide a community of support – giving patients a platform to connect to a digital community for support from professionals and peers, whilst also engaging them in electronically capturing their daily quality of life.
With covid-19 accelerating the need for virtual visits through telehealth, the platform foundations have been laid to give patients access to healthcare professionals whilst in the comfort of their own home. This access removes the need for patients to travel to the clinical trial site for routine follow-up visits. Furthermore, telehealth – in combination with electronic health records – allows patients to stay closer to home even when there is a need for blood draws and/or vital checks which could be done through a local lab.
ENSURING ACCURATE DATA CAPTURE IN A REMOTE SETTING
During a clinical trial, data is key. Whether it is capturing something as simple as how the patient is feeling, to basic vitals (for example, weight or temperature), to dosing, to critical endpoints – all the data needs to be captured, correlated and analysed through the clinical trial process to produce a safe and effective drug. In traditional clinical trial settings, patients would go to a clinical trial site to be dosed, for routine follow-ups and to check vitals. At each touchpoint, data would be captured in a very specific manner by a clinical investigator who had been trained to the clinical protocol. Based on the controls this puts in place, the data is presumed to be accurate.
When considering performing clinical trials in a remote setting, the control over data capture shifts from the clinical investigator to the patient. But even in the hands of the patient, it is imperative to capture both quantitative data points – like when the patient took their medication, how much of the medication was given and vital measurements – and qualitative data points, such as how the patient is feeling from day to day. Through innovative digital technologies, system solutions can be created and integrated to support the full clinical trial ecosystem.
For example, injection pens can be made with sensors for monitoring dose tracking, adherence to the dosing schedule and, in some cases, even progression of the disease state. By enabling the injection pen with the addition of wearable sensors, remote monitoring and electronic patient diaries, you begin to touch on system solutions that can capture the needed data remotely. These passive-data-capture digital solutions can not only capture the same data that would be captured in the clinic but can provide some significant benefits, with a larger quantity of data captured in less time.
According to a Harvard Business School study, if a participant visits a study site a few times a month, the sponsor can collect ~50 hours of data on the participant. Yet the passive collection of patient data in their own home can generate nearly 4,000 hours of data – representing a 75-fold increase. In many respects the data is also more authentic and reliable than that collected in a clinical lab environment. One recent study found that 64% of researchers have used digital health tools in their clinical trials, and 97% plan to use these tools in the next five years.6
EXECUTING A PATIENT-CENTERED SOLUTION
The challenge many clinical trial teams face is taking that first step of accurately defining the needs of the clinical trial and translating them into a system solution customised for that specific trial. Leveraging its core expertise in human-centred design and development, Ximedica is well situated to optimally translate the clinical trial experience to the home setting. Figure 1 shows an aspirational illustration of what that home solution could include.
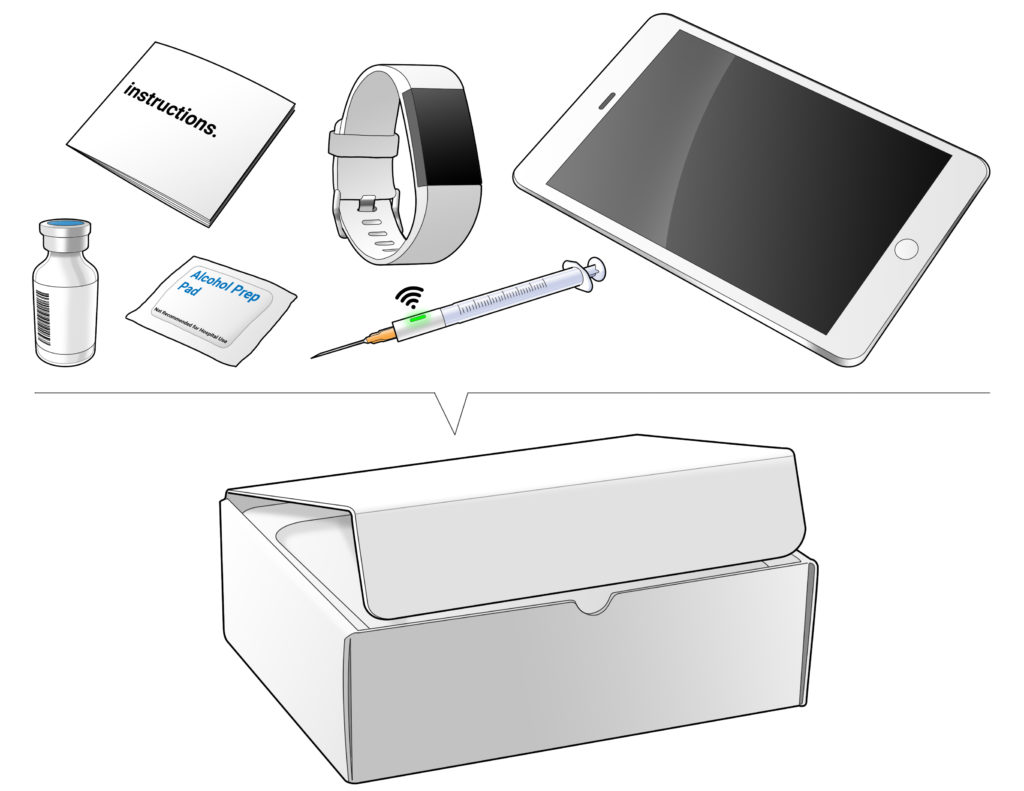
Figure 1: Aspirational illustration of a home setting solution.
To help demonstrate this, Ximedica has broken this problem down into some discrete areas for exploration and concept generation – patient engagement, dosage delivery, data tracking and follow-up, as shown in Figure 2. With each of these areas, there is regulatory consideration as to how it is applied to the solutions for use in a clinical trial and how that may translate into – or conflict with – commercial intent.

Figure 2: Clinical trial engagement concept generation storyboard.
Putting commercial intent aside for the sake of exploring innovative options, Ximedica’s team of engineers has come up with initial solution sets for the previously named categories, including connected/IoT (Internet of Things) smart caps; gamification or progress tracking for continued engagement; involvement of clinician or AI for accountability; in-home-enabled health or progress tracking; dose reminders; passive or minimal extra use steps for dose tracking; and patient interaction or engagement for symptom tracking. In Figures 3, 4 and 5 are some illustrations of what could become part of an overall patient-centric, trial-specific, remote system for clinical trials.

Figure 3: “Charger with Feelings” – Gamification or progress tracking for continued engagement.
While this exercise was aspirational in nature and not limited to one specific clinical trial, the output informs us that the custom solution sets are limitless. With the right strategy and development team in place, a trial-specific solution can be developed to meet the needs of remote clinical trial execution.
DRIVING DOWN CLINICAL TRIAL COSTS

Figure 4: “Weigh the Pen” – Passive or minimal extra use step dose tracking.
By driving patient engagement through enabling digital system solutions to capture patient data in a remote setting, we are aiding patient retention – which decreases the need for additional recruitment. Additionally, connected digital solutions remove the need for on-site patient visits – driving down the per-patient cost. If we are able to decrease the per-patient cost and simultaneously ensure higher patient retention, digital solutions in clinical trials are sure to support the movement to get drugs to market at a lower cost and in less time.

Figure 5: “Voice to Text” – Patient interaction or engagement for symptom tracking.
CONCLUSION
Leaders in biopharma clinical development are experiencing increasingly difficult challenges in screening, retaining and monitoring patients. Now is the time for innovation in clinical trials by seeking a patient-centric approach whilst leveraging the vast array of digital solutions to create a digital clinical trial ecosystem. Ximedica is uniquely poised to deploy game-changing technologies in the clinical trial process and welcomes the opportunity to collaborate and drive improved patient engagement and optimised clinical trial metrics.
REFERENCES
- Rathore A, “Getting A Handle On Clinical Trial Costs”. Clinical Leader, April 25, 2019.
- Coravos A, “Decentralized clinical trials”. Elektra Labs, November 12, 2018.
- “The future of clinical trials”. Research Report, Accenture, September 19, 2019.
- Morgan C, “Are Site-Less Trials the Future of Clinical Research?”. Drug Discovery & Development, September 25, 2017.
- “Update on clinical trials disrupted due to covid-19”. GlobalData Healthcare, May 14, 2020
- Coravos A, “Clinical trials are in need of a digital makeover. Web Page, Harvard Business School’s Digital Initiative, January 23, 2018. (Accessed October 2020)

