Citation: Li B et al, “New Engine for Primary Container Injectors: a Powerful Micro Linear Actuator”. ONdrugDelivery, Issue 117 (Mar 2021), pp 82–85.
Brian Li, Jiunn-Ru Lai, Tsung-Chieh Cheng, Kuang-Hsiang Cheng, Chia-Chi Tina Feng, Min-Ru Wang and Wen-Chi Huang introduce MicroMED’s new engine for primary container injectors, the Primary Actuator, which targets the unmet needs in the autoinjector and wearable injector market as the market shifts towards large injection volumes.
“MicroMED has recently developed a novel microlinear actuator, the Primary Actuator, targeting the unmet needs for the current autoinjector and wearable injector market. The device is specifically designed to drive primary containers, such as a prefilled syringe or cartridge.”
TREND OF CURRENT INJECTOR DEVICES
These days, product development for self-injectable devices is shifting towards large injection volumes. For example, the 2.25 mL autoinjector is one of the epicentres in recent market competition for cutting-edge drug delivery product development, along with many other creative products1,2,3 under development. The other well-known biologics delivery product, the wearable injector,4,5,6 is also moving towards large injection volumes such as 10–20 mL, or even more. Due to this new drug volume shift, the design of the primary containers used within the injectors also needs to be changed accordingly. For example, the prefilled syringe is moving from the ISO standard 1 mL to 2.25 mL, and the prefilled cartridge is moving from ISO 3 mL to 5, 10 and 20 mL. Stiction between the plunger of these large-volume glass containers is one of the key elements to maintain drug sterility, however, many engineering challenges, such as varying injection speed and risk of drug contamination, are associated with this larger container design and more complicated material interactions.
CHALLENGES IN LARGE-VOLUME INJECTION TECHNOLOGIES
Self-administration drug delivery products are limited in driving mechanism options, with springs and motors the most commonly used technologies. Within these driving technologies, spring offers large force and low cost, but comes with problems of varying flow rate (due to decrease of the spring force along with its relaxation) and high storage load (requiring more robust device housing structure making the device much bigger); motor, on the other hand, is usually quite noisy and with a limited driving force.7 To improve device performance, many innovative driving mechanisms have been invented. Volute spring, compressed gas and linear motors are examples of the mechanisms used internally within those large-volume drug-injection devices mentioned above. These state-of-the-art delivery power sources (or “engines”) offer quite large driving force, making it possible to drive large-volume, high-viscosity biologic medication fluids. However, drawbacks such as a bulky footprint and heavier weight still exist with these end products; the use of these engines as the system driving core lowers the convenience and usability of the injector systems for patient and healthcare professionals.
THE ENGINE
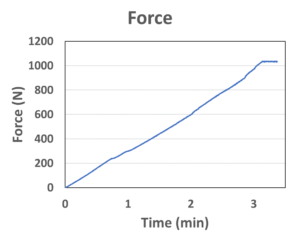
Figure 2: Real-time force output measured by a force sensor. The data saturated at around 1,000 N due to the measurement limit for the sensor, not the limit of the actuator.
MicroMED has recently developed a novel microlinear actuator, the Primary Actuator (Figure 1), targeting the unmet needs for the current autoinjector and wearable injector market. The device is specifically designed to drive primary containers, such as a prefilled syringe or cartridge. This small micro-electromechanical systems (MEMS) engine (22 mm in length, a small but powerful MEMS microchip and micro-integrated control circuitry inside) can provide the injection force/speed for the most challenging specifications required by high-viscosity, large-volume biologics injections. The real-time force output of MicroMED’s actuator was measured using a load cell force sensor (Figure 2). The peak driving force exceeded 1,000 N at around three minutes (the data saturated at around 1,000 N due to the measurement limit of the sensor). The actuator can easily achieve higher force if required, but the 1,000 N force measured already surpasses most of the current driving technologies (springs or motors) by more than 10 times.
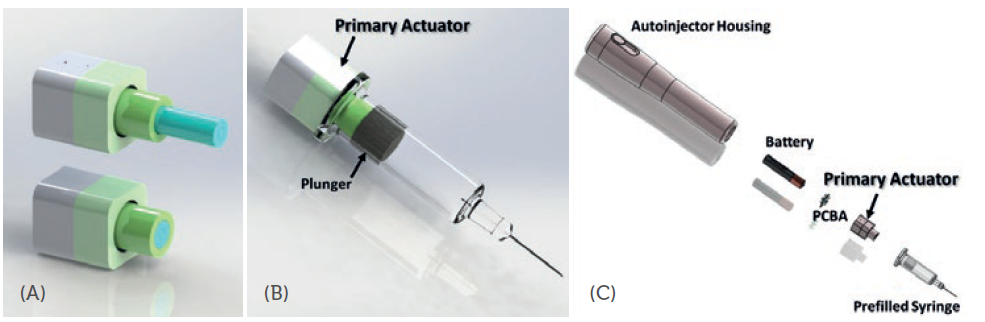
Figure 1: Device 3D photos showing: (a) working principle for the Primary Actuator, (b) an actuator assembled into the space behind the plunger of a prefilled syringe and (c) a simple modulisation design of the actuator with other autoinjector components.
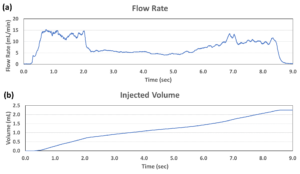
Figure 3: The injection performance (a: flow rate and b: injected volume) of the MicroMED Primary Actuator using a 2.25 mL prefilled syringe with 26G needle and 28 cP viscosity simulated fluid.
MicroMED has also introduced a 2.25 mL prefilled syringe with 28 cP simulated fluid through a 26G needle with injection time of about nine seconds. Figure 3 shows this testing result measured by a commercial flow sensor. The flow rate was stable throughout the whole injection process and the injection volume increased steadily, demonstrating excellent delivery control of the primary drug container. The total capacity needed from the battery was about 5 mAh. This means a small lithium battery would be able to drive the micro actuator, which also helps to lower the overall drug delivery device footprint (with this MEMS actuator as the core engine), making it superior to many current injection driving technologies such as spring, compressed gas and motor (see Table 1 for the performance comparison and Table 2 for the specifications of MicroMED’s engine product).
| Large-Volume Engine Mechanism |
Spring | Compressed Gas |
Micro-Motor | Primary Actuator |
| Maximum Driving Force |
✶✶ | ✶✶ | ✶ | ✶✶✶ |
| Storage Load | ✶ | ✶✶ | ✶✶✶ | ✶✶✶ |
| Varying Injection Speed |
✶ | ✶✶ | ✶✶ | ✶✶✶ |
| Device Size | ✶ | ✶ | ✶ | ✶✶✶ |
| Leakage of Gas | ✶✶✶ | ✶ | ✶✶✶ | ✶✶✶ |
| Drug Contamination | ✶✶✶ | ✶ | ✶✶✶ | ✶✶✶ |
| Noise | ✶✶ | ✶✶✶ | ✶ | ✶✶✶ |
| Modular Design | ✶✶ | ✶✶ | ✶✶✶ | ✶✶✶ |
| Connectivity Integration |
✶ | ✶ | ✶✶✶ | ✶✶✶ |
| Cost | ✶✶✶ | ✶✶ | ✶ | ✶✶ |
Table 1: Comparison of the current driving technologies for the large-volume primary container injectors. Performance scale: ✶✶✶ Excellent, ✶✶ Good, ✶ Normal
| Model # | PA-1W | PA-6W |
| Input Voltage (VDC) | 3.0 | 3.0 |
| Input Power (W) | 1 | 6* |
| Max Load (N)200 | 200 | 1200 |
| Max speed** (mL/min) | 1.5 mL/min | 9 mL/min |
| Stroke (mm) | 35 | 35 |
| Ingress Rating | IP67 | IP67 |
| Weight (g) | 15 | 20 |
| Dimension Body Shaft |
12 x 12 x 22 mm 25 (L) x 9.5 (D) mm |
15 x 15 x 25 mm 25 (L) x 9 (D) mm |
Table 2: Product specifications for the MicroMED Primary Actuator: (a) 1W model and (b) 6W model.
* Customisation engine with up to 24W is also available.
** The speeds were measured using a 2.25 mL prefilled syringe with 1 cP of saline and 30G needle.
The Primary Actuator is an innovative, small, low-cost engine solution for the application of large-volume, high-viscosity medication injection. This engine technology is well suited to address the growing needs for the subcutaneous protein drug therapies. The Primary Actuator has demonstrated its capability as a smart solution for drug delivery that will benefit all stakeholders in the subcutaneous injection market. A successful partnership between the strategic collaborators and MicroMED will enable this engine technology to be developed into a superb commercial injector product meeting the true needs for today’s large-volume biotech injectables.
REFERENCES
- Panting GRF, “Addressing Complex Biologics with Rational Drug DeviceDesign”. ONdrugDelivery Magazine, Issue 105 (Feb 2020), pp 8–12.
- Schneider A et al, “Hold the device against the skin: the impact of injection duration on user’s force for handheld autoinjectors”. Expert Opin Drug Deliv, 2020, Vol 17(2), pp 225–236.
- Simpson I, “The New Emerging Needs Driving Autoinjector Development”. ONdrugDelivery Magazine, Issue 113 (Oct 2020), pp 20–24.
- Bedford T, “Emerging Trends in Wearable Drug Delivery”. ONdrugDelivery Magazine, Issue 111 (September 2020), pp 17–20.
- Shaked T, “Evolution of an On-Body Bolus Injector: From Drug-Specific Device to Platform Technology”. ONdrugDelivery Magazine, Issue 100 (September 2019), pp 20–24.
- De Hann S, “Company Showcase”. ONdrugDelivery Magazine, Issue 100 (September 2019), pp 66–67.
- Fiorini P, “Large-Volume Injectors: Rise in Biologics Brings Challenges”. ONdrugDelivery Magazine, Issue 105 (February 2020), pp 47–50.
Previous article
BUILDING A BETTER PREFILLED SYRINGE FOR COVID-19 VACCINE PACKAGINGNext article
INJAY: SIMPLE BY CHOICE, ADAPTABLE BY DESIGN
