Citation: Agunloye M, Khanolkar A, “Patient-Centricity: the Importance of Human Factors in the Pandemic Era”. ONdrugDelivery, Issue 120 (May 2021), pp 32–38.
Marcus Agunloye and Asmita Khanolkar, Senior Director discuss how patient-centricity is at the heart of drug delivery device design and innovation, and how the global pandemic has highlighted the importance of patient-centric development in this field.
The drug delivery device market is expected to grow tremendously, bringing novel technologies and therapies to life. The key factors driving this innovation peak include a trend towards moving care out of hospitals and into patients’ homes, adoption of smart devices, predictive data analytics, targeted therapeutics and personalised medicine. Patient focus is a common theme in all these drivers. As we begin this decade, it is clear that patient-centricity is at the heart of all innovation and one of the most important considerations throughout the development process. However, there is a lot of work still to be done in this field to truly understand the concept, process and what exactly the success criteria are towards a patient-centric development. The pandemic has opened our eyes to the importance of human factors considerations even further.
MARKET TRENDS
“There is a lot of work still to be done in this field to truly understand the concept, process and what exactly the success criteria are towards a patient-centric development. The pandemic has opened our eyes to the importance of human factors considerations even further.”
Global trends continue to move medical care from hospitals to homes, with the pandemic threat of covid-19 the push for virtual care, longer time between hospital visits, and at-home care for chronic diseases has increased. An example is the transfer of chemotherapy treatment from hospitals to homes, which has led to a requirement for subcutaneous delivery of cancer treatments as opposed to intravenous delivery in a hospital setting. This trend is generating a need for devices for self-administration that can deliver large volumes of formulation and drug containers that can withstand high pressures in order to provide acceptable delivery times. Successful high-pressure delivery device systems will determine the success of this important transition from hospital to home.
Smart devices are an area of opportunity to enhance user engagement with their treatment process. Using a smartphone or other digital device can offer the patient an expanded user interface, including useful functionality such as dose tracking, reminders and training information, and can also offer opportunities for the prescriber to understand adherence when care is moved to the home. However, this relies on two things from the patient – access to technology and their engagement with the smart system. Whilst the adoption of smartphones is becoming more and more widespread, there are still patient populations that may struggle to afford an up-to-date smartphone, so understanding patient access to technology must be a key consideration in the inclusion of this type of technology into a drug delivery device.
Whilst adding further functionality and data information for the user through a connected device may seem like an easy way to improve the patient experience, it is important to understand if the patient wants to engage with this. Some patient populations may find the steps required to set up a connected device confusing or taxing, or may have a general reluctance towards technology that might make a connected device a barrier to adherence rather than a motivator. Overall, there may be compelling use cases for the integration of digital systems into drug delivery devices, but it is important to understand how the patient population will respond to it by exploring the area with them and considering the full range of costs and benefits associated with this type of integration before moving forward with it.
“The three effects of the covid-19 pandemic, namely isolation, virtual care and remote learning, are all elements that amplify the problems of self-administered devices.”
HUMAN FACTORS CONSIDERATIONS IN THE PANDEMIC
Human factors considerations are even more important than ever in identifying specific patient needs, particularly in the current global pandemic. The three effects of the covid-19 pandemic, namely isolation, virtual care and remote learning, are all elements that amplify the problems of self-administered devices. Under the conditions of the pandemic, patients couldn’t be trained in hospitals and overall access to healthcare practitioners (HCPs) was limited, yet more and more therapies started moving towards home care. The pandemic emphasised the need for intuitive devices that need only very minimal training. Crisis devices are often used by a carer, a teacher or even a passer-by, and again, in such cases, intuitive-to-use devices are needed for effective dosing.
In addition, the effect of isolation and emotion on the use of the device requires a non-threatening, welcoming, integrated functional approach. The pandemic also highlighted the effects of disparity where technology may not be universally available. Not everyone is technology savvy, and digital literacy may be a big factor in the correct use of the device. In addition, diversity factors are more exaggerated with remote site limitations, spread of information and patient involvement. It is clear that the one-size-fits-all approach does not work with diverse patient populations, including paediatric and geriatric populations, and a range of users from patient to carer to community pharmacist, and a variety of dose regimens are required.
ELEMENTS OF A “PATIENT-CENTRIC” MODEL
The four important areas of considerations for human factors include patient safety, patient outcomes, patient adherence or compliance, and finally, the overall patient experience (Figure 1). Patient safety and therapy outcomes are the first layers of consideration. Current complaints vary with the application and include both crisis and chronic use. For life threatening diseases, obviously this can be profoundly serious and have serious consequences. For emergency-use devices, it is imperative that the accurate dose is injected at the correct depth, intramuscularly or subcutaneously. Some of the human factors considerations in these applications focus around patient populations. Patient physiology is different in children compared with adults, and important considerations of needle-to-muscle depth accuracy repeatability when overcoming population diversity is an important factor.
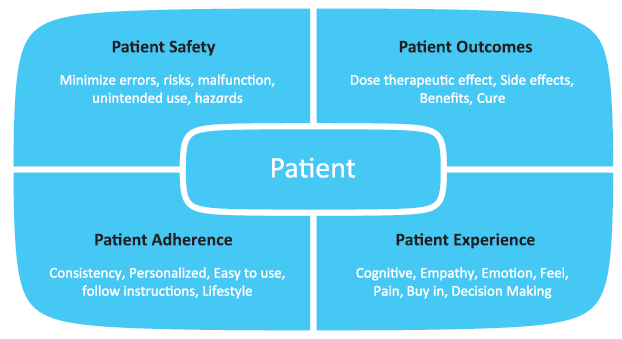
Figure 1: Elements of a patient-centric model.
“Using patient insights on cognitive and emotional needs, lifestyle, population diversity, technology access and adoption skills, reliability and sustainability can help add further benefits to the therapy.”
Some of the challenges with glass containers include breakage and repeatability issues due to siliconised rubber. In high-pack glass syringes, patients have experienced variability of injection times, resulting in wet injections. In the case of biologic drugs, this can potentially cause immunological response due to wet injections. Other impurities such as tungsten in needle-staked glass containers can pose issues for the stability of delicate drugs, such as biologics, and result in degradation of the drug and unwanted therapy outcomes. Finally, use errors are on the rise due to the pandemic and lack of training, this demands intuitive devices.
Patient adherence and overall experience are the second layers of considerations and equally important. Particularly with self-administered devices, ease of use becomes a priority. Devices need to be easy to use without training, or with minimal training, should promote adherence, be non-intimidating and perform robustly and reliably every time for a positive overall experience.
Long-acting injectables provide a better overall patient experience due to a reduced number of injections. However, it is important to focus on adherence and if the patient will remember to administer their injection regularly each month, and to create a feedback loop so the prescriber knows that the patient is receiving their treatment. Training decay can also be an issue if the patient only uses the autoinjector once a month – will they be able to remember how to use it again a month later?
First and foremost, the best approach to solve this is to make the device as simple and intuitive to use as possible. This can also be supported with training the patient, such as having an HCP guide the patient through the first few injections and by having robust information available for the patient to support themselves, such as training videos and clear instructions for use.
A patient-centric model requires additional elements with the patient at the heart of the thought process, and designing around the patient. Using patient insights on cognitive and emotional needs, lifestyle, population diversity, technology access and adoption skills, reliability and sustainability can help add further benefits to the therapy. Oval’s approach to device development combines a deep understanding of the patient’s needs, coupled with the technical needs when developing patient-centric autoinjectors that can be customised to deliver a wide range of drug formulations, including fragile molecules for both subcutaneous and intramuscular injection with high viscosities and a wide range of delivered volumes (Figure 2).
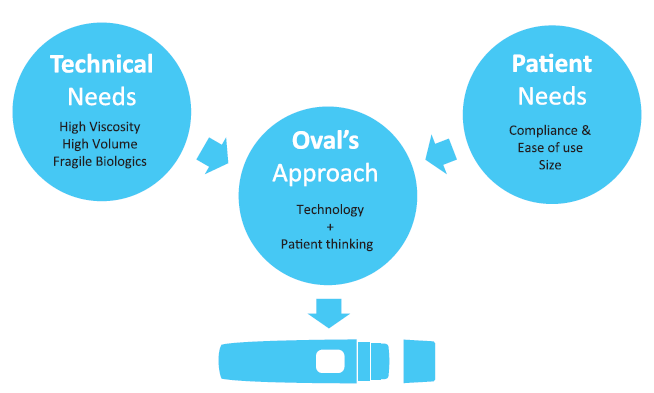
Figure 2: Incorporating patient needs for intuitive autoinjector designs.
PATIENT-CENTRIC DESIGN THINKING
To put the user case at the heart of the process, the device design must satisfy functional, cognitive, aesthetic and emotional balance. This requires flexibility in the design space for both the inside and outside of the device. Moulded primary drug containers provide both flexibility of container size and shape, and control of tolerances for reliability of repeated use. The inside mechanics need to deliver the therapy in optimised delivery time, depth and bolus size.

Figure 3: Key design features with a human approach.
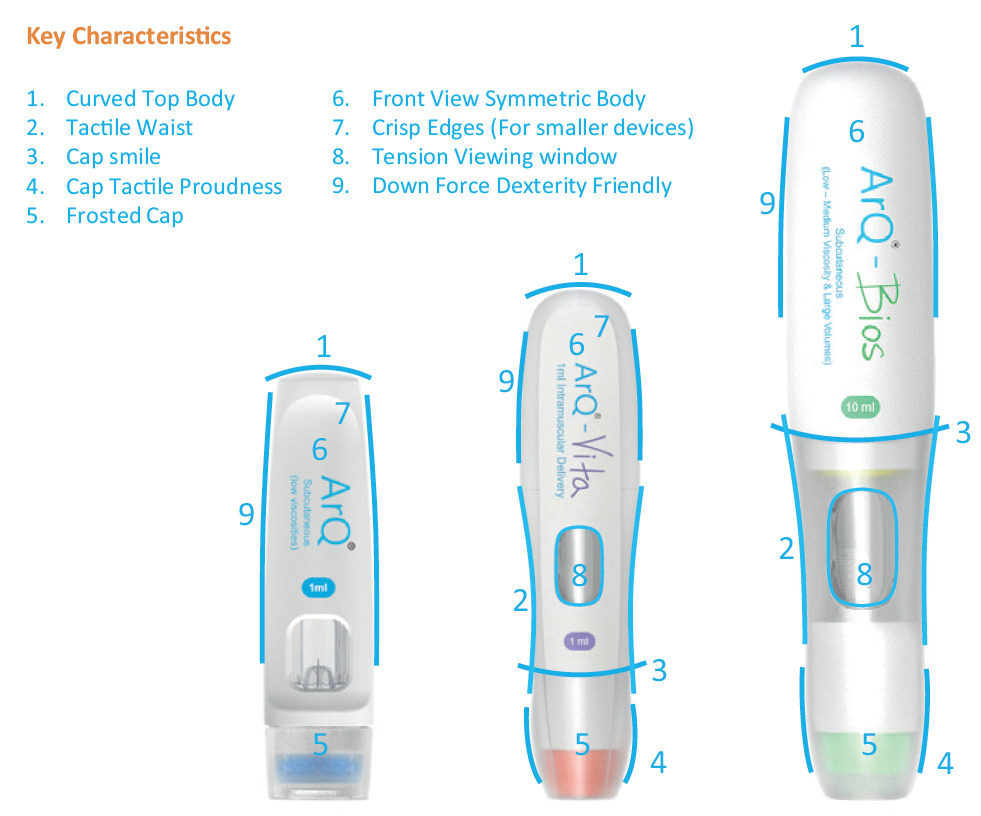
Figure 4: Human factors attributes applied to design.
The outside is equally important for key design features with a human approach, such as those shown in Figure 3. Figure 4 shows the human factors attributes applied to the product design.
The combination of innovative design coupled with small footprint, quiet devices that provide consistent delivery performance for patient safety, outcome, adherence and preference is a crucial consideration.
PATIENT PROCESS MAPPING
Patient process mapping is a method that outlines the process of how the autoinjector is used, such that the inputs of the three elements that affect device use – the user, the use environment and the user interface – are captured in the process. Early-stage field work is a must to put the patient’s needs to the forefront at each stage of autoinjector design.
A key focus in the early stages is getting feedback and input from patients and users as soon as possible. Through fieldwork, such as interviews with patients, caregivers and HCPs in their living and working environments, insight into the patient’s treatment journey can be gained. This can be used to map out each of the touchpoints the patient interacts with during their treatment, identifying pain points and difficulties they may encounter along the way. From this, the patient’s needs throughout the process can be identified, taking into account the basic need to receive the dose, along with their emotional needs related to their treatment, and an understanding of issues with treatment that may become barriers to adherence (Figure 5).
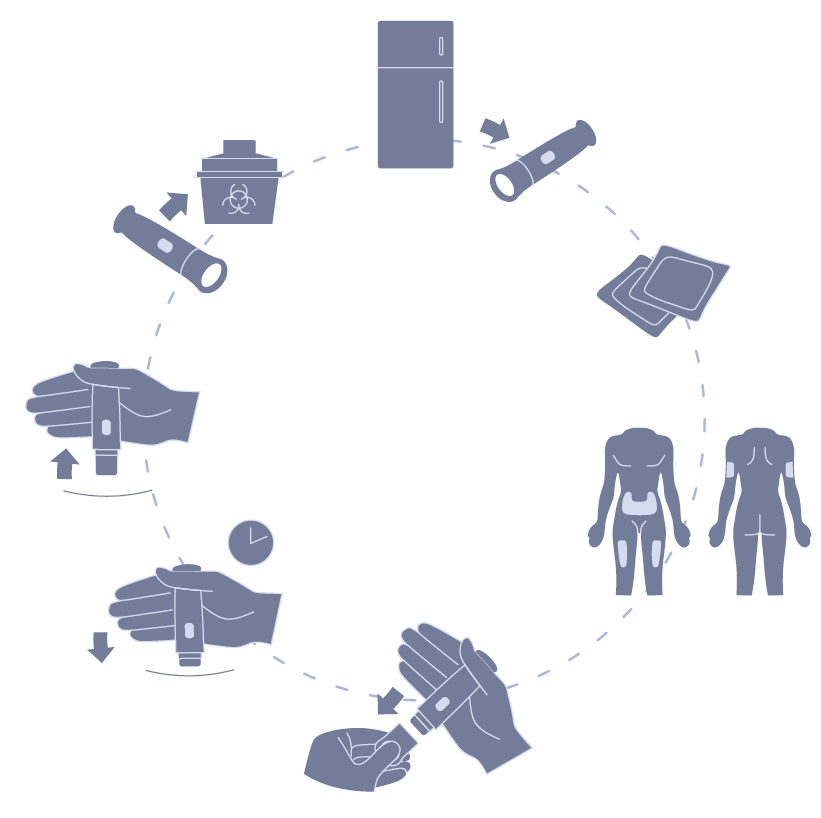
Figure 5: Patient process mapping.
“Patient input will always be key to developing the best possible autoinjector. By involving the patient in the process early and often, you have the best chance of uncovering their full range of needs and ensuring that what you are designing responds to them.”
There are many examples of such early-stage insights that can have a real effect on the design approach of an autoinjector. Chronic versus crisis applications pose different requirements. Looking at a crisis autoinjector that patients must always carry with them, Oval found users adopting a range of coping strategies for how to store their devices when carrying them day to day – they were using anything from pencil cases to zip-lock bags to Tupperware containers in attempts to make sure that their life-saving device was protected and ready in case they needed to use it. This pointed to an unmet need – many users didn’t feel their autoinjector was safe enough in the packaging materials they were provided with by the manufacturer – and one that may not have been uncovered in a formative evaluation in the development of the autoinjector, as few users vocalised these feelings until they were directly asked how they were currently carrying their autoinjector.
A range of packaging concepts were developed at the same time as the crisis autoinjector. Concepts were selected and prototyped, giving a range of materials that could be brought to an early-stage formative evaluation, provoking further conversations with users about their full range of needs from end to end of their treatment process, while at the same time developing an understanding of how they were able to use the device.
This approach can be applied to both platform customisation and bespoke device development, with early-stage user research occurring in parallel with the drug formulation characterisation process, providing a strong understanding of both key inputs: user needs and technical needs. From there, with detailed device-user input, supporting materials can be developed, while the internals of the device are developed to meet the needs of the drug formulation. Depending on the identified patient needs, a number of design solutions may be developed encompassing device packaging and labelling details, with a view to bringing one or more concepts to an early-stage formative evaluation.
PATIENT-CENTRIC DEVELOPMENT STRATEGY
These early-stage activities then form a crucial input for the rest of the human factors process. Early concepts are refined and narrowed down and, as the autoinjector design develops, subsequent formative evaluations can be undertaken with increasingly high-fidelity prototypes. This feedback loop of design development and user interaction allows both the development of a thorough understanding of the users’ interactions with the device and use-related risk, while also continuing a conversation with the patient and user population about their needs beyond just the delivery of their medication (Figure 6).
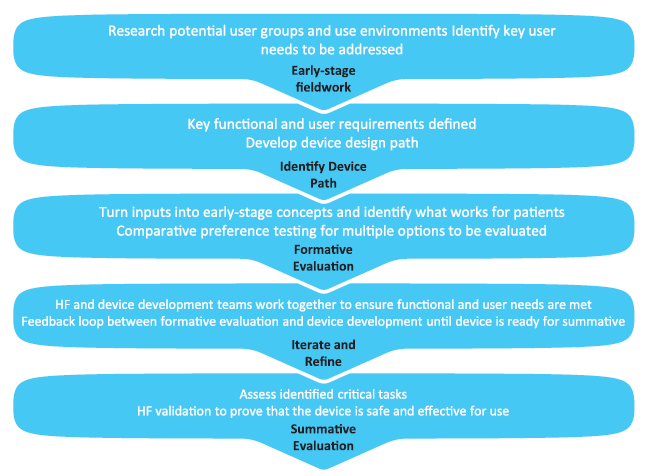
Figure 6: Human factors process.
As the device reaches maturity, a thorough understanding of the use of the device user interface and its associated risks has been developed, with mitigations applied where necessary, ready for validation. At the same time, this process maintains the user and patient’s voice throughout, putting focus not just on the device but on the wider environment and scenarios that affect the patient’s experience, resulting in an end product that is both safe and effective for the user, and optimised to the needs of the patient throughout their treatment journey.
PATIENT USABILITY STUDIES
Formative usability studies are an important part of the feedback loop in patient-centric development, providing insights into device handling and patient preference. The use steps of the device can be tested, with prototype variants of the device provided with packaging, labelling and instructions for use, allowing the evaluation of injection site preference, observation of use errors for safety, improvement areas for the user interface, effectiveness of training and population diversity factors. Examples of feedback measurements may include successful dose delivery and an understanding of the visual and audible feedback provided by the device. The feedback from these studies can then be used to inform device design, developing a device that users can understand and feel comfortable using.
Late-stage user studies can then be used to further analyse the design of the user interface once it has matured. Through the use of tools such as use-related risk analysis, critical tasks can be identified, and, by testing these with the intended user population, it can be determined if the device is safe and effective for use and ready for human factors validation. Alongside this, late-stage user studies can also be used to verify that the design decisions made in response to early-stage observations work for the patient population’s wider needs through discussion on the use of the device.
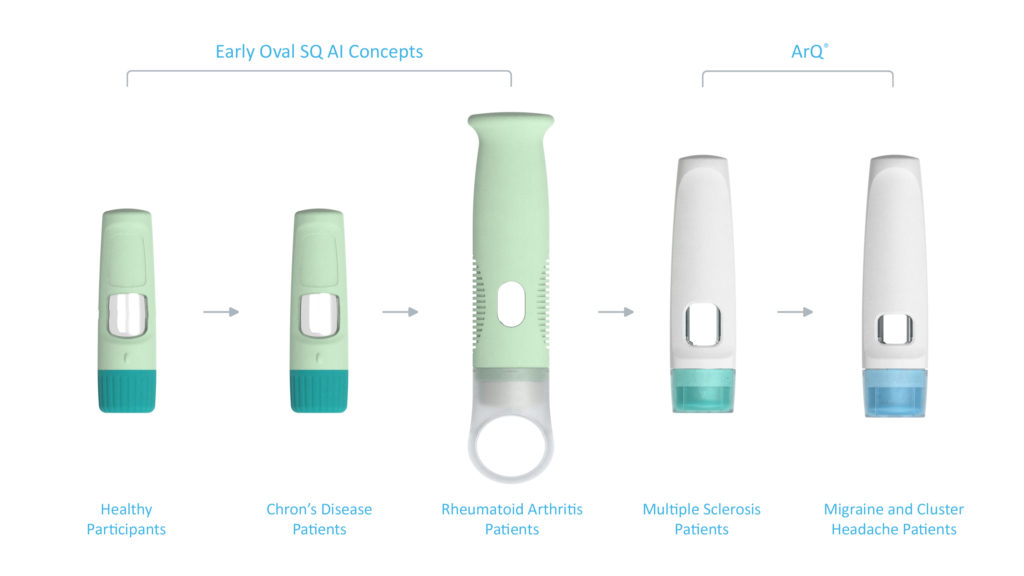
Figure 7: Design evolution through multiple user studies and patient populations.
This approach was taken in the case study shown in Figure 7, where three user studies were conducted on early-stage device designs. The results from these studies eventually led to the design of the ArQ platform, with its user interface undergoing further refinement through another two user studies (Figure 8).

Figure 8: Optimised user interface design elements for ArQ.
Through this process, findings were made, both about how the device functions and how it fits into the patient’s daily life. In the early stages, user feedback led to the development of the key user interface elements, now found across Oval’s platforms, such as the audible and visual feedback mechanisms. The process also involved testing an autoinjector variant tailored to patient populations with low manual dexterity, the learnings from which influenced Oval’s approach to building an understanding of patient’s specific needs and customising devices to patient populations (Figure 9).

Figure 9: Optimised user interface design elements for ArQ-Bios high viscosity/volume.
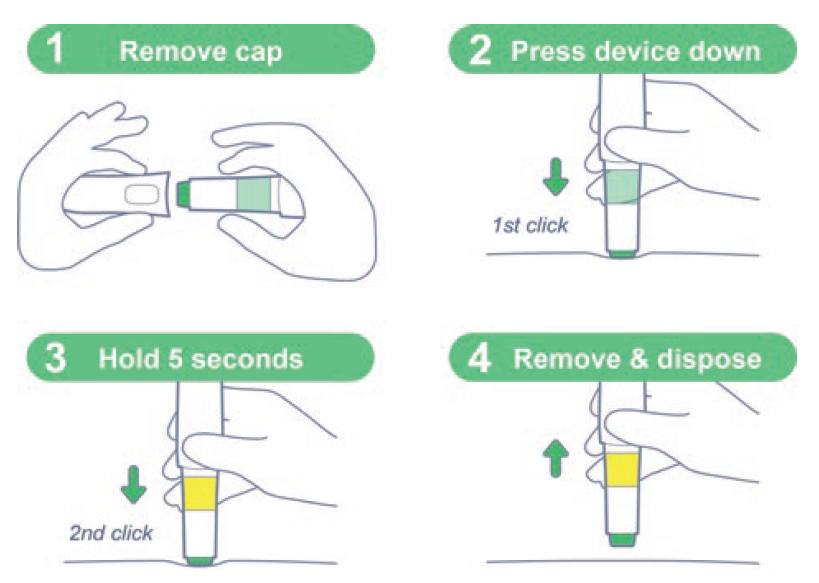
Figure 10: “Easy-to-use” steps for a patient-centric design.
PATIENT FEEDBACK
Patient input will always be key to developing the best possible autoinjector. By involving the patient in the process early and often, you have the best chance of uncovering their full range of needs and ensuring that what you are designing responds to them. By making sure this input is implemented throughout the design process, you can reach validation with a device that is not just safe and effective to use but fits properly into the patient’s life and eases some of the issues they may have previously experienced with their treatment (Figure 10).

