Citation: Vico A, Barichello E, “How to Ensure the Future Demand for Biologics Can Still be Delivered by Injectables”. ONdrugDelivery, Issue 120 (May 2021), pp 8–12.
Anthony Vico and Enrico Barichello discuss the demands that high-viscosity biologic formulations place on prefilled syringes, and how pharmaceutical companies can make the most of this growing market segment to deliver improved quality of life for patients.
Global biologic sales are expected to grow from a market size of US$325 billion (£230 billion) in 2020 to $425 billion (£300 billion) by 2024. That represents an estimated compound annual growth rate (CAGR) of 7%, and a significant opportunity for pharmaceutical companies.1
“Managing a chronic condition can place a high demand on patients, often requiring regular visits to a clinical setting to receive their therapies, frequently via intravenous methods. Self administration via the subcutaneous route relieves a great deal of this burden by handing greater control to the patient.”
Biotech drugs, especially monoclonal antibodies (mAbs) and recombinant proteins, have enabled the pharma industry to treat incurable critical and chronic diseases, such as osteoporosis, rheumatoid arthritis and hypercholesterolemia, meeting previously unmet needs and delivering better outcomes for pharma companies and patients in equal measure. Because most biologics are primarily administrated parenterally, particularly via the subcutaneous route, prefilled syringes (PFSs) have gained strong acceptance as the preferred delivery system.
Market trends have led pharma companies to formulate concentrated biologics that reduce the required frequency of injections for those who suffer from chronic conditions. These complex biologics are characterised by high chemical sensitivity and strong dynamic characteristics (i.e. viscosity), which pose significant challenges to PFS technology. As a leading provider of primary packaging and drug delivery systems, Stevanato Group is consistently challenging itself to deliver best-in-class, optimised solutions to improve patients’ lives.
This article reviews the current state of the PFS market and discusses the challenges associated with biologic drug delivery, before covering a case study on how to optimise needle design to mitigate a patient’s discomfort perception while guaranteeing acceptable administration performance for higher viscosity medicine. Finally, this article considers the key drivers in the choice of PFS, advocating an early-phase, full-system approach to mitigate risk and speed time to market for these life-enhancing therapies.
The burden of chronic disease continues to weigh heavily on populations across the globe. In Europe alone, these illnesses are the leading cause of mortality by far, accounting for 77% of the total disease burden and 86% of all deaths.2 Cross-referencing these statistics with demographic trends, such as increasing life expectancy, has further implications for the future of healthcare provision.
By 2100, the proportion of those aged 65 years and over is projected to increase. This is despite forecasts that the overall population across the EU will decline in comparison with 2019 levels. As such, this age group will grow its share from a fifth of the current total (20.3%) to almost a third (31.3%),3 equating to an additional 39.7 million people who are at risk of living with some form of chronic disease, or even multiple chronic diseases, over the coming decades.
Given the damaging impact of these conditions on citizens’ wellbeing and the associated negative consequences for economic health, it is understandable that prevention and early detection of chronic disease is a clear focus among policymakers, who are seeking to tackle the issue as close to its source as possible.
At the same time, there is a growing requirement to address chronic diseases from the patient’s perspective through long-term management and sustained treatment regimes. Pharmaceutical companies are pursuing innovative R&D programmes in these areas that, with supply chain partners, aim to uncover new treatments and drug delivery techniques that will ease many of the obstacles facing patients and healthcare professionals.
“When dealing with biologics bringing these benefits to the patient is not necessarily as straightforward as it sounds. The main sticking point being the high viscosity of the formulations in question.”
THE RISE AND RISE OF BIOLOGICS
Biologics are at the forefront of these developments. Between 2014 and 2018, the share of net medicine spending on biologics grew from 30% to 42% of the total. Over this same period, the Center for Drug Evaluation and Research (CDER) within the US FDA approved the biologic licence applications (BLAs) for 59 novel biologics. This more than doubles the 26 approved in the preceding five-year period.4 A further 10 biologics were approved in 2019, as well as 10 biosimilars based on previously approved therapeutic biological products.5 Cancer and orphan diseases (indications affecting 200,000 patients or fewer) are the most targeted therapeutic areas.
Since parenteral delivery is the typical route of administration, the demand for syringes is predicted to rise in line with the growth in biologics. Forecasts suggest this will result in the global PFS market expanding at a CAGR of 9% from 2020 to 2025, when it will be valued at $8.6 billion.6
There are several trends that are driving the uptake of biologic treatments delivered via PFSs, one key example being patient convenience. Managing a chronic condition can place a high demand on patients, often requiring regular visits to a clinical setting to receive their therapies, frequently via intravenous methods. Self-administration via the subcutaneous route relieves a great deal of this burden by handing greater control to the patient.
However, adherence remains an obstacle to effective treatment, particularly where preparation is required using a vial and syringe. In comparison with vials, PFSs deliver a ready-made, high-concentration dose that has been prepared to precise tolerances. These benefits mean medication errors and overfill waste are reduced and adherence is increased, with the potential for further improvements in adherence when used in conjunction with autoinjectors and as part of a connected combination product platform.
SOLUTIONS FOR HIGH VISCOSITY
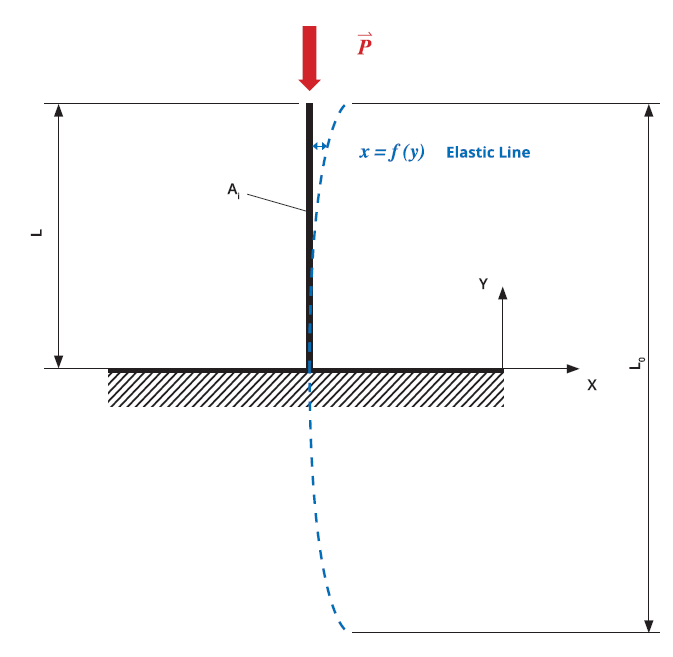
Figure 1: Theoretical predictive model based on Euler’s critical load analysis, simulating the application of axial load on the needle tip to cause hook deformation.
When dealing with biologics, however, bringing these benefits to the patient is not necessarily as straightforward as it sounds. The main sticking point is the high viscosity of the formulations in question. This is due to the strength of the intermolecular forces at play in highly complex, long-chain biologics, such as MAbs, combined with the fact that protein concentration is deliberately formulated very high in order to meet dosing requirements. While viscosity is seen to increase moderately in low-concentration formulations, in high concentration formulations of greater than 100 mg/ml, viscosity increases exponentially.7
At higher viscosities, difficulties can arise for parenteral delivery mechanisms, including the need for additional force to be applied to the plunger rod. This can mean injections take longer and some traditional or more conventional autoinjector technologies may be rendered unfit for purpose if the force required is too high. Solving this challenge typically requires compromise on the part of the patient, such as increasing the frequency of injections, and/or when it comes to the PFS, such as increasing the needle gauge or selecting specific low-friction plunger stopper components; both scenarios have clear drawbacks in terms of compliance, comfort and ease of use. It has been down to innovators in the field of primary packaging and drug delivery systems to find a pathway that satisfies patient expectations from the perspective of both biologic drug delivery and user experience.
Stevanato Group, as a leader in this field, has carried out a detailed analysis of design developments intended to manage these issues. Its work looked closely at the morphological features of thin-walled needles, which offer the advantage of simultaneously maintaining flow rates and reducing needle gauge. However, they also increase the risk of both needle-tip deformation (e.g. hooks) and coring (the formation of elastomer particles caused by the needle tip piercing the elastomeric needle shield). The research identified that needle-tip deformation is a product of the reduced mechanical strength of the cross-section. In parallel, Stevanato Group confirmed that coring propensity is linked to aspects including the sharpness and geometry of needle-tip bevels, the mechanical characteristics of the elastomeric needle shield, and the tribology between the needle tip and the elastomeric needle shield – all of which are critical to quality.
In a further study, Stevanato Group attempted to determine how varying the needle-tip geometry would mitigate the risk of deformation and coring propensity when moving to a thin-walled configuration. This involved comparing the geometries of a five-bevel and a three-bevel needle tip. The research investigated the mechanical performance of the two designs, examining their hooking propensity in the context of the required penetration force.
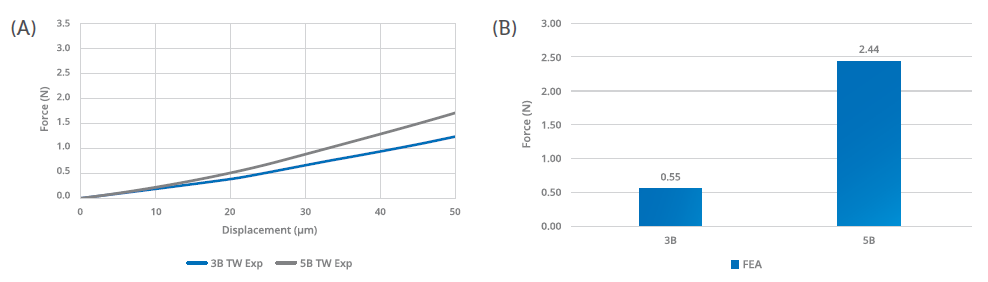
Figure 2: The finite element analysis confirmed a significantly lower hooked needle propensity for the five-bevel needle tip design compared with the three-bevel needle tip design. The trend (A) and external load (B) required to generate a hooked needle at 50 μm is significantly higher – approximately five times – for the five-bevel needle tip design than with the three-bevel needle tip design.
The study used theoretical finite element analysis (FEA) predictive models to determine the difference between the two needle-tip designs in terms of hooking propensity. Both models were based on the experimental test setting, with a vertical load applied to control deformation. The theoretical prediction model was based on Euler’s critical load analysis, simulating an axial load applied to the needle tip to cause hook deformation (Figure 1). Based on this model, the five-bevel needle design withstood an axial load 83% higher than the three-bevel needle design before achieving the same deformation magnitude (50 μm), as shown in Figure 2. This finding was confirmed by the numerical predictive model, which indicated that the axial load required to arrive at the same deformation magnitude (50 μm) would be 555% higher for the five-bevel needle than the three-bevel needle.
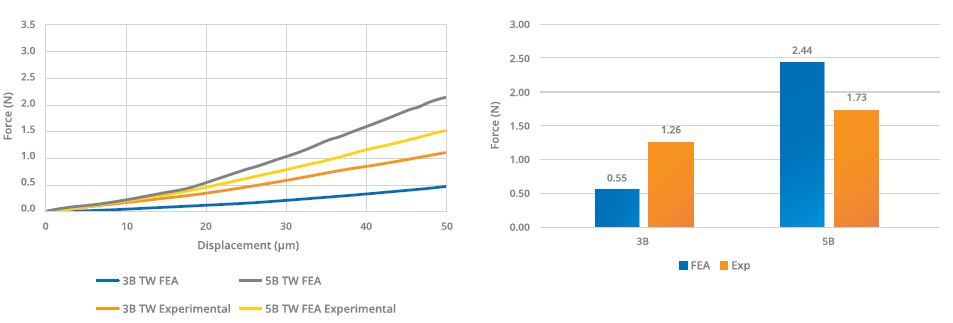
Figure 3: Comparison between numerical and experimental hooking propensity testing.
The five-bevel needle’s lower propensity for hooking was further underlined by subsequent experimental testing. This testing also demonstrated that the predictive models overestimated the performance gap between the needle tips (Figure 3). To analyse the penetration force, the different needle types were inserted into a specific fixing tool and moved at constant speed towards a plastic substrate (Figure 4). The insertion force was recorded as a function of penetration depth through a calibrated load cell, with the five-bevel needle registering a slightly lower needle penetration force (median of 1.12 N) compared with the three-bevel needle (median of 1.12 N). The conformance (variance) was found to be consistent between the two needle designs.
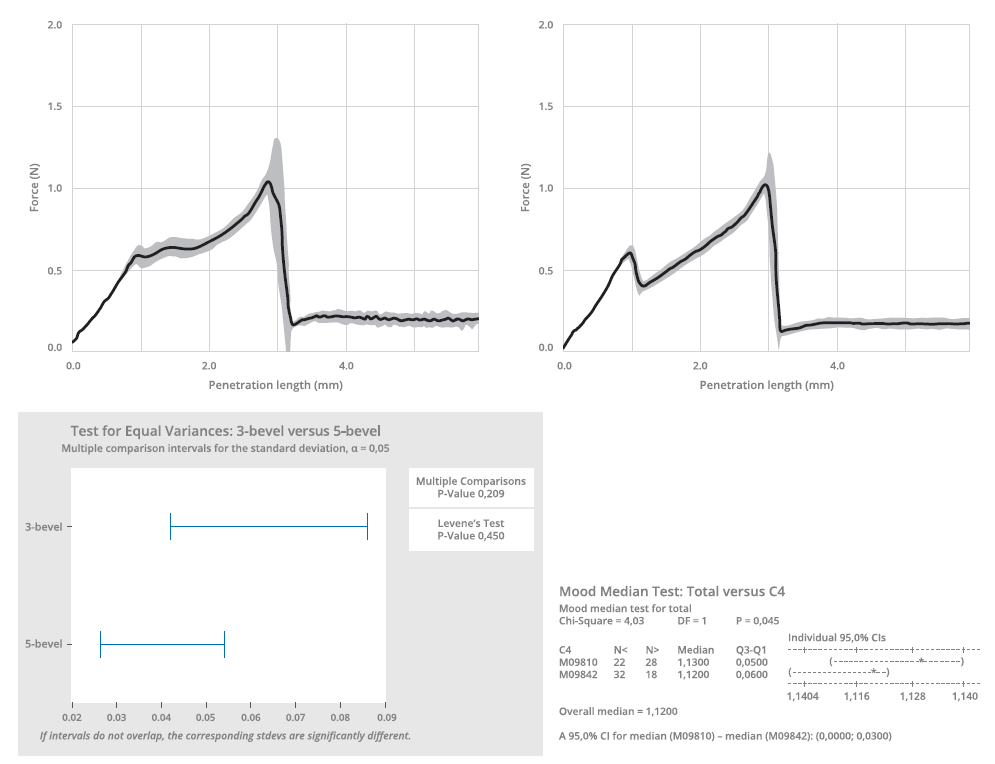
Figure 4: A penetration force test was performed to compare the three-bevel and five-bevel needle (thin walled). The penetration test is described by ISO 7684.
“Stevanato Group’s SG Alba® and SG Nexa® product lines, for example, have been designed specifically to meet the requirements of high-value biologics, accommodating even large-volume (2.25 mL) high-viscosity formulations.”
MATCHING PFS CHARACTERISTICS WITH FORMULATION
From a device design perspective, one of the clear takeaways from Stevanato Group’s research is the importance of factoring primary container selection into the development process for injectable drugs. The ultimate end goal is to achieve a unified solution that incorporates all aspects of the container closure system and device at the desired tolerances and performance levels. This is dependent on a range of analytical techniques that must be used to understand the interplay between the various elements, which in turn provides the knowledge base to optimise the choice of container for the specific drug product in question. If considered in the early stages of a project, this ensures the overall process is as streamlined as possible, avoiding potential disruption further down the line.
The need to bring specialist knowledge to the table early in proceedings underlines the importance of the relationship between project owners and supply chain stakeholders. Stevanato Group understands this dynamic, and is focused on its mission to provide its partners with a comprehensive suite of systems, processes and services that guarantee medicine integrity.
Stevanato Group’s SG Alba® and SG Nexa® product lines, for example, have been designed specifically to meet the requirements of high-value biologics, accommodating even large-volume (2.25 mL) high-viscosity formulations. This is due to their high dimensional and geometrical accuracy, as well as enhanced properties relating to glide ability, frictional fluid dynamics and extractables profiles. In addition, both product lines can be easily integrated into active and passive needle safety systems and into automatic drug delivery devices, such as spring-based autoinjectors.
These qualities ensure that Stevanato Group can de-risk the development process for its pharma partners, saving costs and accelerating product time to market. However, the ultimate stakeholder in this process is, of course, the patient. Chronic conditions affect the lives of millions of people and Stevanato Group’s research serves as a reminder of the need to continually evaluate the injection experience with a view to reducing the discomfort that patients must repeatedly endure over an extended period. By continually identifying ways to advance needle technology, and by presenting this in a convenient, patient-friendly proposition – one that integrates syringe, needle closure and device – Stevanato Group will continue to help pharmaceutical partners deliver the day-to-day patient benefits that, together, add up to an enhanced quality of life.
REFERENCES
- Stops A, Karanis Y, MacDonald D, “Trends in Auto-Injectors. Early considerations for an emergency use auto-injector development and manufacturing: a case study”. CPhI webinar series, Feb 2021.
- “Chronic Disease & Policy”. European Chronic Disease Alliance, web page.
- “Population projections in the EU”. Eurostat, Sept 2020.
- de la Torre BG, Albericio F, “The Pharmaceutical Industry in 2019. An Analysis of FDA Drug Approvals from the Perspective of Molecules”. Molecules, 2020, Vol 25(3), p 745.
- “Biosimilar Product Information”. US FDA, web page.
- “Prefilled Syringes Market by Type [Conventional (Disposable, Reusable), Safety], Material (Glass, Plastic), Design (Single-Chamber, Dual- Chamber, Customized), Application (Diabetes, Cancer, Arthritis, Anaphylaxis, Ophthalmology) – Global Forecast to 2025”. Markets and Markets, Sept 2020.
- Palm T et al, “The Importance of the Concentration-Temperature- Viscosity Relationship for the Development of Biologics”. BioProcess International, Mar 2015.
Previous article
THE ADDED VALUE OF A PLATFORM APPROACH: PEN INJECTOR CASE STUDYNext article
DRIVERS FOR CHANGE IN ASEPTIC AUTOMATION
