Citation: Costa A, “Enhanced Process Control for Lipid-Based Nanoparticles: LNPs and Liposomes”. ONdrugDelivery, Issue 123 (Aug 2021), pp 44-47.
Antonio Costa discusses the use of lipid nanoparticles in the modern drug delivery industry and introduces the DIANT system for the continuous processing of nanoparticles with integrated process analytical technology, which can provide the quality required for these injectable, complex formulations.
INTRODUCTION
Nanoparticles are once again in the spotlight, this time as a critical tool to combat the spread of covid-19. In particular, messenger ribonucleic acid (mRNA) encapsulated in lipid nanoparticles (LNPs) has been successfully manufactured, distributed and administered to populations worldwide. The high efficacy and strong protection of covid-19 mRNA-LNP vaccines has generated significant interest in them as a candidate for future vaccines and therapeutics. However, information released by the EU EMA covid-19 data leak emphasises the need to better understand quality issues observed in the LNP manufacturing process – for example, quality issues may be traced to RNA instability and LNP particle size.
“Liposomal nanoparticles have been approved as drug products throughout the world for a variety of clinical indications, including cancer and fungal treatments, with the highest percentage of liposomal drug products being anti-cancer therapies.”
mRNA-LNPS
The components of mRNA-LNPs can be divided into four main groups:
- An ionisable, cationic lipid
- One or more neutral/structural lipids
- mRNA
- Buffering systems and water.
All of these components come together to form a sophisticated and tunable delivery system. In this case, the delivery cargo is mRNA and the target delivery location is a specific tissue or cell type. There is a truly remarkable relationship between all of these components that have made LNPs a success story. To start, the mRNA, which has a particular sequence of nucleotides, is processed by ribosomes to synthesise a protein, such as a spike protein.
The ionisable, cationic lipid has multiple roles, such as promoting the encapsulation of mRNA during the LNP synthesis process and releasing the mRNA within the cytoplasm. The neutral/structure lipids, such as distearoylphosphatidylcholine (DSPC) and cholesterol, provide the general framework or structure of the particles, whereas pegylated lipids coat the surface of the LNPs and provide physical stability and enable longer circulation times in the host.
Buffering systems are important for properly forming and neutralising the particles. Additionally, water, a sometimes-overlooked component, is very important in the core structure of the LNP, providing a continuum of hydrogen bonds between the mRNA and the hydrophilic portions of the lipid components. Accordingly, the LNP formulation is a critical factor linked directly to RNA encapsulation and multiple aspects of the delivery system’s overall pharmacokinetics and pharmacodynamics, including the localised target tissue or cellular expression.
“Scalable processing and manufacturing technology platforms are still being developed.”
LIPOSOMES
Liposomal nanoparticles have been approved as drug products throughout the world for a variety of clinical indications, including cancer and fungal treatments, with the highest percentage of liposomal drug products being anti-cancer therapies. The APIs in these drug products are typically small molecules such as doxorubicin, daunorubicin, cytarabine or irinotecan.
The basic principle of how liposomes are effective is that they deliver a high payload directly to the site of action, such as a tumour, resulting in a more efficacious and safer drug product. In brief, liposomes consist of a lipid bilayer that surrounds an aqueous compartment. The majority of liposomes that have been approved by regulatory agencies have lipid compositions that include phosphatidylcholine lipids, cholesterol and either a PEGylated or charged lipid.
The composition of the lipid bilayer plays a major role in the liposome’s stability, both in storage and in vivo conditions, meaning that the type of lipids and their molar ratios are considered critical material attributes. For example, cholesterol is known to affect bilayer rigidity and PEGylated lipids promote steric stabilisation of the bilayer. The API can either go into the lipid bilayer or be encapsulated in the aqueous compartment. Moreover, targeting moieties can be added to the lipid bilayer to provide a direct targeting mechanism in vivo.
A major benefit of liposomal drug products is that they greatly reduce the side effects that occur with non-liposomal chemotherapy treatments. In addition, liposomes promote longer blood circulation times, resulting in a longer half-life of the API. Although these therapeutics are very beneficial to patients, scalable processing and manufacturing technology platforms are still being developed.
“By using the NanoFlowSizer and DIANT system’s feedback algorithms, a highly reproducible and consistent particle size can be achieved from the beginning to the end of the processing run.”
CURRENT MANUFACTURING METHODS
Current manufacturing methods to produce lipid-based nanoparticles include solvent injection, homogenisation and microfluidic mixing. Solvent injection methods are typically performed by pre-mixing ethanol with a lipid and then injecting the mixture into an aqueous phase. Solvent injection methods comprise crossflow injection, static-mixers, microfluidic mixing and turbulent jet technologies, with the latter providing a highly controllable mixing strategy. Microfluidics offer a variety of mixing techniques and various geometries have been developed to mix the solvent stream with the aqueous stream.
Each of these strategies rely on different degrees of convective and diffusive forces for mixing. For example, microfluidics may mix in a very low Reynolds number state (laminar flow), whereas crossflow injection, t-mixers and turbulent jets operate at much higher Reynolds number states (turbulent flow).

Figure 1: DIANT LF system for nanoparticle synthesis, part of the DIANT production series.
CONTINUOUS PROCESSING OF NANOPARTICLES
DIANT Pharma has introduced a series of systems that use a continuous manufacturing technology and implement a turbulent jet in co-flow. Integrated process analytical technology (PAT) provides enhanced control from particle formation throughout downstream processing, which includes ultrafiltration/diafiltration (UF/DF), purification and bioburden reduction. The DIANT system is a scalable, turnkey solution that uses only a single jet, producing particles from 0.2 LPM up to 100 LPM (Figure 1).
The scalability of this technology is an important consideration when selecting processing equipment, from lab to commercial scale. The DIANT system has multiple modules and is offered as a continuous, closed system. The add-on modules include a buffer exchanger/concentration module, an active loading/surface modification module and a final purification stage/bioburden reduction module. The system can be a hybrid system, running in single unit operation or batch mode, or as an integrated system in an end-to-end continuous manufacturing process.
SR-DLS for Automatic Particle Size Control
One of the PAT tools integrated in the DIANT systems is the NanoFlowSizer (Figure 2), a spatially resolved dynamic light scattering (DLS) technology developed by InProcess-LSP (Oss, Netherlands). This PAT tool is highly useful for measuring multiple attributes of the particles being processed, such as the Z-average (d.nm) particle size, polydispersity index (PDI), turbidity and particle size distribution (PSD). Attributes such as the Z-average, PDI and PSD are typically listed in product specifications and may be considered critical quality attributes for liposomes and LNPs.

Figure 2: NanoFlowSizer, an important PAT tool used to provide on-line or in-line particle size data.
Spatially Resolved DLS (SR-DLS) technology was incorporated over other DLS techniques as it enables particle size analysis at high flow rates, such that the particle size can be analysed on-line and/ or in-line, as opposed to at-line or off-line. The on-line/in-line approach supports a rapid assessment of the particle size and directly measures the particles in the process stream without the nanoparticles going to waste or requiring sample handling by an operator. There is also the major benefit in that the NanoFlowSizer flow cells can be cleaned or steamed in place.
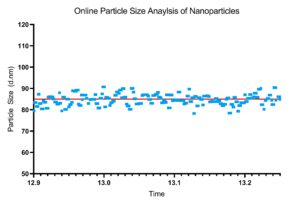
Figure 3: Example particle size data for a 30-minute run.
Enhanced Control Using the DIANT System
By measuring the particle size on-line or in-line, the particle size measurements can be used as a feedback mechanism to control the formation of the nanoparticles. When considering that nanoparticle formation is highly susceptible to process condition changes, the added layer of protection using a feedback mechanism offers much-needed quality control. Running the system for extended durations with inevitable subtle changes occurring in the local environment can impact the overall process on the nano-level. By using the NanoFlowSizer and the DIANT system’s feedback algorithms, a highly reproducible and consistent particle size can be achieved from the beginning to the end of the processing run (Figure 3).
Research and Development System – A Low Flow Starting Point
The DIANT LARU is a lower flow continuous processing system using the same jet technology found in the DIANT production series (Figure 4). This system is designed to have a low dead volume to reduce material costs, such as when working with costly lipids or mRNA. The DIANT LARU is offered with multiple add-ons, such as an in-line particle size module and a tangential flow filtration module.

Figure 4: DIANT LARU, a research and development system for nanoparticle synthesis, scalable to the DIANT production series.
CONCLUSION
LNPs have a bright future in both the vaccine and therapeutic space. However, to make the most of their potential a scalable, high-throughput processing technology is required to move smoothly from lab to commercial scale. The DIANT system, integrated with NanoFlowSizer, provides the enhanced control required to consistently produce uniform and high-quality nanoparticles.

