Citation: Righton L, “Six Inhaler Sustainability Myths – and Why They Must be Busted”. ONdrugDelivery, Issue 126 (Oct/Nov 2021), pp 30–34.
Louise Righton busts six sustainability myths about inhalers.
“The NHS has identified the inhaler market as one area of decarbonisation to help reach its net-zero target, citing that pMDIs account for 3% of all emissions.”
The 26th UN Climate Change Conference of the Parties (COP26) is being held in the UK from October 31 to November 12, 2021. The COP26 summit will bring parties together to accelerate action towards the goals of the Paris Agreement and the UN Framework Convention on Climate Change.1
Countries are being asked to come forward with ambitious 2030 emissions reductions targets that align with reaching net zero by the middle of the century. The UK was the first country to pledge to reduce carbon emissions by 78% by 2035.2 The UK NHS stated its aim to be the world’s first net zero national health service for the emissions it controls directly, aiming to become net zero by 2040, with an ambition to reach an 80% reduction by 2028 to 2032.3
It is recognised that healthcare is a significant contributor to the global carbon footprint – this has been calculated to be equivalent to 4.4% of net emissions in 2014.4 While more than half of these emissions are from energy use, medicines also contribute; inhalers, particularly pressurised metered-dose inhalers (pMDIs), have come under scrutiny due to the environmental impact of the propellants used. While this scrutiny is positive because it will encourage change, much of the debate is narrowly focused on the comparative global warming potential (GWP) of different device types. As a result, advocacy too often provides blanket recommendations that amount to “device switching” – the notion that propellant-free devices, such as dry powder inhalers(DPIs) and soft mist inhalers (SMIs), are categorically superior to pMDIs, regardless of patient considerations (Figure 1).
Figure 1: Switching has the potential to create additional emissions and further costs to the NHS.7
For example, the NHS has identified the inhaler market as one area of decarbonisation to help reach its net-zero target, citing that pMDIs account for 3% of all emissions.3 This has led the NHS to put prescribing targets in place to promote the rapid uptake of DPIs by switching patients from pMDIs in the non-salbutamol market. This is to be achieved by financially incentivising prescribers. From October 2021, the Investment and Impact Fund (IIF) is rewarding increased prescribing of DPIs and SMIs where clinically appropriate, such that by 2023–2024, only 25% of non-salbutamol inhalers prescribed will be pMDIs.5 This is a significant reduction from current usage patterns – in the UK, 70% of all inhalers prescribed are pMDIs,6 which includes a significant 61% rate of pMDI prescribing in the non-salbutamol category.7
“For a complex medical challenge, pMDIs are a crucial option for physicians. The literature does not support the myth that pMDIs have a role to play solely in delivering SABA therapy.”
This focus on reducing the carbon footprint of the inhaler market by switching from pMDIs to alternative devices may have unintended negative consequences for the NHS’s net-zero ambition.7 For all inhaler types, there is an impact on the environment to consider at each stage of the inhaler lifecycle, from manufacturing, supply and usage by the patient to appropriate disposal when the labelled number of doses have been taken or the inhaler is no longer needed.8 A recent paper produced by the International Pharmaceutical Aerosol Consortium (IPAC), which was established to represent the industry in navigating the Montreal Protocol in the late 1980s, and of which Kindeva (formerly 3M Drug Delivery Systems) was a founding member, proposes that the most effective approach to the global environmental challenge surrounding inhalers is for healthcare systems to adopt a holistic, patient-outcome-based approach. This approach would aim to reduce the carbon footprint of patients using inhaled treatments while simultaneously supporting improvements in patient care and reducing the environmental impact of medical treatments at all stages of the lifecycle.9
Kindeva Drug Delivery has a legacy of advancing the environmental sustainability of inhaled medicines. A pioneer in hydrofluoroalkane (HFA)-based formulations for pMDIs, Kindeva developed the world’s first chlorofluorocarbon (CFC)-free pMDI (launched in 1995). This innovation was a milestone accomplishment that delivered step-change improvements in the environmental impact of inhaled drugs, and Kindeva remains at the forefront of environmental progress today. The pMDI industry is making great strides in the transition to low GWP propellants, with HFA-152-a from Koura (Cheshire, UK) and Honeywell’s (NC, US) HFO-1234-ze(E) the current candidates to replace existing propellants HFA-227ea and HFA-134a.10
Nevertheless, despite the industry’s commitment to decarbonisation, in the form of investment in reformulation and manufacturing technology,11–15 a narrative has evolved that pMDIs are inherently “bad” and must be replaced as quickly as possible by alternative inhaler types. There are several flaws in the argument that the solution to the carbon footprint of respiratory disease is to be found in a rapid switchover from pMDIs to DPIs. Therefore, the prevailing “myths” must be examined and addressed to achieve the shared industry vision of a decarbonised inhaler market.
Myth 1: For Most Patients, DPIs Are More Effective Than pMDIs, and Patients Prefer Them
Proponents of the “pMDI to DPI switch” method of decarbonising the inhaler market will often selectively cite studies that seemingly prove that DPIs are more effective than pMDIs and that patients prefer them. This myth is leveraged to promote a rapid switch from pMDIs to DPIs in the non-salbutamol segment. The pMDI is then pigeon-holed solely as a solution for reliever medication (short-acting beta agonist, or SABA). In fact, the relative efficacy of different types of device – particularly pMDI compared with DPI – has been studied extensively by researchers for decades. Systematic reviews and meta-analyses show no significant differences in effectiveness across devices in general.16–18 Recent head-to-head randomised controlled trials and population studies show a set of applications for which pMDIs are currently the device proven to be significantly more effective, or at least as effective, as DPIs.19–24 For a complex medical challenge, pMDIs are a crucial option for physicians. The literature does not support the myth that pMDIs have a role to play solely in delivering SABA therapy.
Myth 2: Patients Should be Made Aware of the Carbon Footprint of Their Inhalers and be Encouraged to Switch From pMDIs for Environmental Reasons
Matching the patient with the right inhaler is a complex decision that the clinician must judge and should not be overly carbon led. Guidelines from the Global Initiative for Asthma and the Global Initiative for Chronic Obstructive Lung Disease emphasise this.
Asthma and chronic obstructive pulmonary disease (COPD) patients with stable disease should have continuity of inhaler device.25 Suddenly asking the patient to switch from a device that is working to a new device for carbon-led reasons, not medical reasons, can have negative consequences: patients can lose confidence in their treatment,26,27 they can suffer a reduced perception of disease control28 and they can even lose trust in their health practitioner and health system.29 Empirical findings and best practice suggestions consistently advise against switching away from established, functioning treatments without a clear, clinical objective.
“There’s no “one-size-fits-all” inhaler; treatment must be tailored from the plethora of options available, and patients should be engaged in the decision.”
Myth 3: A Minority of Patients and Some Young Children are Unable to Use DPIs, but the Majority of Patients Find Them Easier to Use as They Require Less Co-ordination Than a pMDI, Leading to Greater Adherence
An essential element of optimised disease management is the training and correct usage of devices.30 While the handling of all pMDIs requires the same approach, different types of DPI pose different challenges to patients.31 The baseline rate of correct use of inhalers is low, with the literature suggesting that up to 94% of DPI users and 74% of pMDI users make mistakes.32 Beyond correct use, overall adherence to inhaled medicines is low, with 60% of COPD patients and up to 70% of asthma patients non-adherent to their prescribed therapy.33–35 Some previous studies show pMDIs are associated with better disease control and treatment adherence among subjects with asthma and that they have equivalent treatment satisfaction to DPIs.36
Incorrect usage can be particularly problematic for patients who are prescribed multiple DPIs because it can be challenging to train patients to use devices with different designs – some incorporating reservoirs of powder, others requiring single-use loading with a capsule, for example. It has been suggested that physicians should avoid prescribing multiple devices requiring different handling and dosing techniques.37 In short, there’s no “one-size-fits-all” inhaler; treatment must be tailored from the plethora of options available, and patients should be engaged in the decision.
Myth 4: pMDIs are the Only Inhaler Type With an Environmental Impact and Should be the Focus of Sustainability Policies
Propellants are only one part of the story – we need a shared understanding of each product’s lifecycle and its total environmental impact so that we can expand the discussion from a single focus on GWP to a more holistic approach to sustainability. The industry-led CFC-HFA transition made major improvements in the sustainability of pMDIs, and facilitated the introduction of more DPIs, expanding patient and clinician choice. With a wider choice of devices on the market, an environmental policy must go beyond propellants and consider other raw materials used in the drug product or device, how they are sourced, how easily they are recycled and where they go after the patient has finished using them. In the quest to reduce GWP, we must not overlook environmental impacts such as human toxicity, fossil depletion and marine eutrophication. A recent study found that an HFA-152a pMDI inhaler has the lowest impacts for 10 out of 14 environmental categories considered, while the DPI is the worst option for eight impacts.38
The development of pMDIs with low GWP propellants will raise the bar on sustainability, and manufacturers and stakeholders must rise to the challenge of sustainability through the product and patient lifecycle. We have an opportunity as an industry to address the wider sustainability picture and broaden the thinking beyond propellants.
Myth 5: Another Advantage of DPIs is that they have Dose Counters, so the Patient Knows How Many Doses are Left and Doesn’t Throw Away a Part-Full Inhaler, Thereby Minimising Wastage
The majority of pMDIs now use a dose counter to ensure minimal wastage. At the forefront of sustainability thinking at Kindeva is a reduction in the parts count of plastic devices. For example, the company is developing a new metal-free dose counter with far fewer parts than has been available previously. Kindeva believes this new dose counter delivers benefits throughout the value chain – the simplicity of assembly, compatibility with a broad range of actuators and valve types and the robust reset of dose counting – and also represents a marked sustainability improvement by reducing plastic use. The industry must recognise that these improvements are possible through the regular cycles of innovation, and it must continue to challenge itself on sustainability.
Myth 6: To Decarbonise the Inhaler Market Quickly, we Need to Switch as Many Patients as Possible From pMDIs to DPIs, Thus Reducing the Carbon Footprint of Each Patient and Achieving Net Zero
A recent study found that the NHS’s rapid switchover policy is unlikely to result in a substantial reduction in carbon emissions, compared with the no policy implementation alternative, and will likely result in rising costs of treatment and an adverse impact on patient health (Figure 2). The study included an academic literature review that suggests that about 3.7% of switched patients will have one additional exacerbation resulting from the switch, leading to additional hospitalisation and GP appointments, and use of a reliever inhaler, all creating additional emissions and further costs to the NHS.7
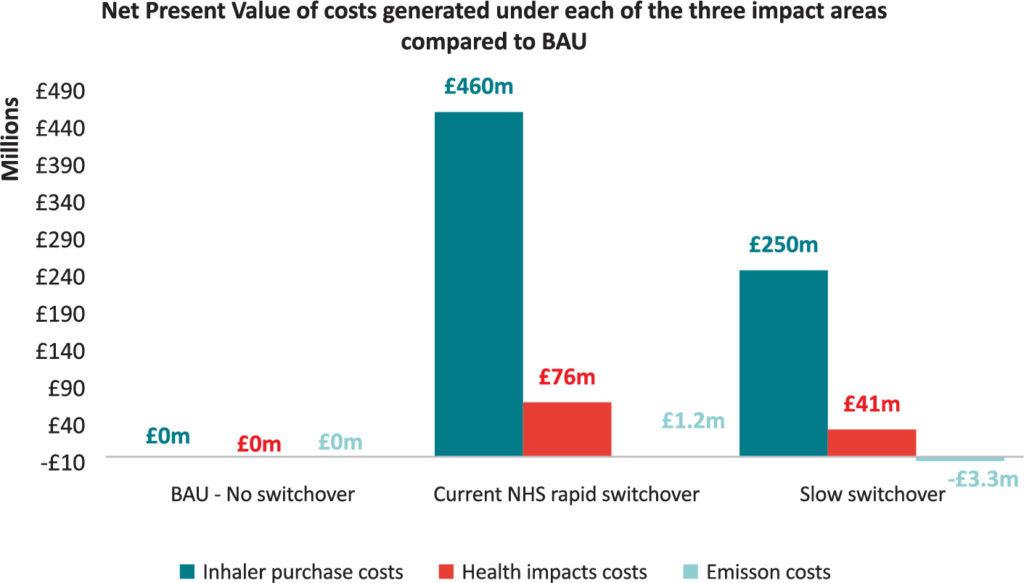
Figure 2: Assessing the economic impact of the NHS’s pMDI to DPI switch policy versus business as usual and a slower switchover.7
CONCLUSION
In order to ensure a smooth transition to low GWP pMDIs, and to maintain the pMDI as a key delivery platform that many patients and prescribers rely on, there are a number of unhelpful “myths” surrounding the sustainability of inhalers, which have become the prevailing narrative and which must be countered. The industry is fully committed to introducing pMDIs containing low GWP propellants as soon as is practicable, and stakeholders need to support this aim. Current policy to reduce pMDI prescribing in the non-salbutamol segment may result in the unintended consequence of slowing down the introduction of new propellants across both the non-salbutamol and the salbutamol segments, the latter being reliant upon pMDIs. Robust sustainability strategies require a holistic, end-to-end view of product design, lifecycle analysis and patient impacts. Decarbonising the inhaler market cannot be optimally achieved by a rapid reduction in pMDI prescribing – a more holistic view must be adopted for the good of the environment, patients and health systems.
REFERENCES
- Web Page, 26th UN Climate Change Conference of the Parties (COP26), Glasgow, UK. (https://ukcop26.org, Accessed September 27, 2021.)
- Web Page, COP 26 Explained. (https://2nsbq1gn1rl23zol93eyrccj-wpengine.netdna-ssl.com/wp-content/uploads/2021/07/COP26-Explained.pdf, accessed September 27, 2021)
- “Delivering a ‘Net Zero’ National Health Service”. PDF, NHS, Oct 2020.
- “Health care climate footprint report”. Health Care Without Harm. Accessed December 20, 2020.
- “Primary Care Networks, Network Contract DES, Investment and Impact Fund. Annex B – Investment and Impact Fund: 2021/22 and 2022/23”. NHS England, August 24, 2021, updated October 1, 2021. (https://www.england.nhs.uk/wp-content/uploads/2021/08/B0828-iii-annex-b-investment-and-impact-fund-21-22-22-23.pdf, Accessed October 8, 2021.)
- Janson C et al, “Carbon footprint impact of the choice of inhalers for asthma and COPD”. Thorax. 2020, Vol 75(1), pp 82–84.
- Bar-Katz V, Woolley NJ, Vitello E, “Economic Analysis of NHS England’s Policy to Reduce the Carbon Impact of Inhalers by Encouraging a Rapid Prescribing Switch from Pressurised Metered Dose Inhalers (PMDIs) to Dry Powder Inhalers (DPIs)”. Value in Health, 2021, Vol 24(12, S2). Ahead of publication (due in December 2021).
- Lewis H, Hardwick M, Rignall A, “A Multi-Stakeholder Approach to Minimising the Environmental Impact of Inhaled Therapies and Improving Patient Care”. Respiratory Drug Delivery, 2021, Vol 1, pp 45–56.
- “A multi-stakeholder approach to minimising the environmental impact of inhaled therapies and improving patient care. A proposal for discussion”. The International Pharmaceutical Aerosol Consortium. Accessed January 4, 2021.
- Needham M, Cocks P, Righton L, “Next-Generation Pressurised Metered Dose Inhalers: a Holistic, Patient-Centred Approach to Sustainability”. ONdrugDelivery Magazine, Issue 112 (Sep/Oct 2020), pp 10–13.
- “Chiesi outlines €350 million investment”. Press Release, Chiesi, November 4, 2019.
- “AstraZeneca’s ‘Ambition Zero Carbon’ strategy to eliminate emissions by 2025 and be carbon negative across the entire value chain by 2030”. Press Release, AstraZeneca, Jan 22, 2020.
- “Kindeva Drug Delivery Announces Knowledge Transfer Partnership with Loughborough University to Study Sustainable Propellants”. Press Release, Kindeva DrugDelivery, May 20, 2021.
- “Kindeva Drug Delivery Announces Collaboration with Monash University to Study Sustainable Propellants”. Press Release, Kindeva Drug Delivery, Jun 10, 2021.
- “GSK announces major renewable energy investment and low carbon inhaler programme alongside Life Sciences sector Race to Zero ‘breakthrough’ at NYC Climate Week”. Press Release, Sep 20, 2021.
- Brocklebank D et al, “Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature”. Health Technol Assess. 2001, Vol 5(26), pp1–149.
- Ram FS et al, “Systematic review of clinical effectiveness of pressurised metered dose inhalers versus other hand held inhaler devices for delivering β2agonists bronchodilators in asthma”. BMJ, 2001, Vol 323(7318), pp 901–905.
- Dolovich MB et al, “Device selection and outcomes of aerosol therapy: Evidence-based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and Immunology”. Chest, 2005, Vol 127(1), pp 335–371.
- Wittbrodt E et al, “Differences in health care outcomes between post discharge COPD patients treated with inhaled corticosteroid/ long-acting β2-agonist via dry-powder inhalers and pressurized metered-dose inhalers”. Int J Chron Obstruc Pulmon Dis, 2019, Vol 14, pp 101–114
- Jones R et al, “The comparative effectiveness of initiating fluticasone/ salmeterol combination therapy via pMDI versus DPI in reducing exacerbations and treatment escalation in COPD: a UK database study”. Int J Chron Obstruct Pulmon Dis, 2017, Vol 12, pp 2445–2454.
- Ming SWY, Haughney J, Ryan D, “P274 Comparison of the initiation of COPD treatment with licensed FDC ICS/LABA treatments in terms of disease control and cost effectiveness”. Thorax 2017, Vol 72, A232.
- Muraki M et al, “Which inhaled corticosteroid and long-acting β-agonist combination is better in patients with moderate-to-severe asthma, a dry powder inhaler or a pressurised metered-dose inhaler?”. Drug Deliv, 2017, Vol 24(1), pp 1395–1400.
- Hojo M et al, “Comparison of the clinical effects of combined salmeterol/fluticasone delivered by dry powder or pressurized metered dose inhaler”. Pulm Pharmacol Ther, 2016, Vol 37, pp 43–48.
- Kerwin E M et al, “Pharmacokinetics, pharmacodynamics, efficacy, and safety of albuterol (salbuterol) multidose dry-powder inhaler and ProAir® hydrofluoroalkane for the treatment of persistent asthma: results of two randomized double-blind studies”. Clin Drug Investig, 2017, Vol 36(1), pp 55–65.
- Bjermer L, “The importance of continuity in inhaler device choice for asthma and chronic obstructive pulmonary disease”. Respir, 2014, Vol 88(4), pp 346–352.
- Ding B et al, “Maintenance inhaler preference, attribute importance, and satisfaction in prescribing physicians and patients with asthma, COPD, or asthma–COPD overlap syndrome consulting for routine care”. Int J Chron Obstruct Pulmon Dis, 2018, Vol 13, pp 927–936.
- Small M et al, “Importance of inhaler-device satisfaction in asthma treatment: real-world observations of physician-observed compliance and clinical/patientreported outcomes”. Adv Ther, 2011, Vol 28(3), pp202–212.
- Björnsdóttir US, Gizurarson S, Sabale U, “Potential negative consequences of non‐consented switch of inhaled medications and devices in asthma patients”. Int J Clin Prac, 2013, Vol 67(9), pp 904–910.
- Doyle S et al, “What happens to patients who have their asthma device switched without their consent?”. Prim Care Respir J, 2010, Vol 19(2), pp 131–139.
- Usmani OS, “Choosing the right inhaler for your asthma or COPD patient”. Ther Clin Risk Manag, 2019, Vol 15, pp 461–472.
- Chrystyn H et al, “Device errors in asthma and COPD: systematic literature review and meta-analysis”. NPJ Prim Care Respir Med, 2017, Vol 27(1), p 22.
- Lavorini F et al, “Effect of incorrect use of dry powder inhalers on management of patients with asthma and COPD”. Respir Med, 2008, Vol 102(4), pp 593–604.
- Laube BL et al,”What the pulmonary specialist should know about the new inhalation therapies”. Eur Respir J, 2011, Vol 37(6), pp 1308–1331.
- Lindsay JT, Heaney LG, “Nonadherence in difficult asthma – facts, myths, and a time to act”. Patient Prefer Adherence, 2013, Vol 7, pp 329–336.
- Elliott RA, “Poor Adherence to Anti-inflammatory Medication in Asthma: Reasons, Challenges, and Strategies for Improved Disease Management”. Dis Manag Health Out, 2006, Vol 14(4), pp 223–233.
- Aggarwal A, Kumari R, Grover S, “Treatment Satisfaction With Metered Dose and Dry Powder Inhalers in Patients With Bronchial Asthma”. Chest, 2016, Vol 150(4), 13A.
- Rootmensen GN, “Predictors of incorrect inhalation technique in patients with asthma or COPD: a study using a validated videotaped scoring method”. J Aerosol Med Pulm Drug Deliv, 2010, Vol 23(5), pp 323–328.
- Jeswani HK, Azapagic A, “Life cycle environmental impacts of inhalers”. J Clean Prod, 2019, Vol 237, 11773.

