To Issue 127
Citation: Suman J, Karavas N, “Delivering on the Promises of Intranasal Vaccination”. ONdrugDelivery, Issue 127 (Nov 2021), pp 10–14.
Julie Suman and Nektaria Karavas review intranasal delivery as a method of vaccine administration and consider the challenges and benefits of intranasal delivery.
“The respiratory nature of SARS-CoV-2 has led to greater interest in nasal drug delivery as a method for tackling the virus.”
As dawn broke on January 1, 2021, people around the world awoke with the hope that the new year would bring a greater level of protection against SARS-CoV-2, the virus behind the covid-19 pandemic.
At that point in time, the proportion of the globe’s population to have received any dose of a covid-19 vaccine stood at an almost negligible 0.07%, but, within months, the picture had changed dramatically. Following the intensive roll-out of national vaccination programmes, more than 5.5 billion vaccine doses have now been administered, and this figure is increasing by more than 30 million doses every day as the covid-19 immunisation programme continues worldwide.1
These staggering numbers show just how quickly the pandemic moved vaccines from the background of everyday life to the forefront of public consciousness, underlining their critical importance to the health and wellbeing of people the world over.
“In terms of its anatomical and physiological structure, the highly vascularised lateral wall of the nasal cavity provides a welcome environment for drug molecule absorption.”
One of the knock-on effects has been a dramatic disruption to the value of the global vaccines market. Worth an estimated US$33 billion (£24.3 billion) prior to the pandemic, growth projections multiplied within months of covid-19 surfacing on the world stage.2 Today, the WHO reports that pharma revenues from covid-19 vaccines alone could have the potential to reach $150 billion over the 2021–2022 period.3
Within this highly active space, more than 3,300 vaccines, involving 415 different indications, are currently in development, including both therapeutic and prophylactic varieties (Figure 1). While injection remains the dominant delivery in the vaccine space, the respiratory nature of SARS-CoV-2 has led to greater interest in nasal drug delivery as a method for tackling the virus.
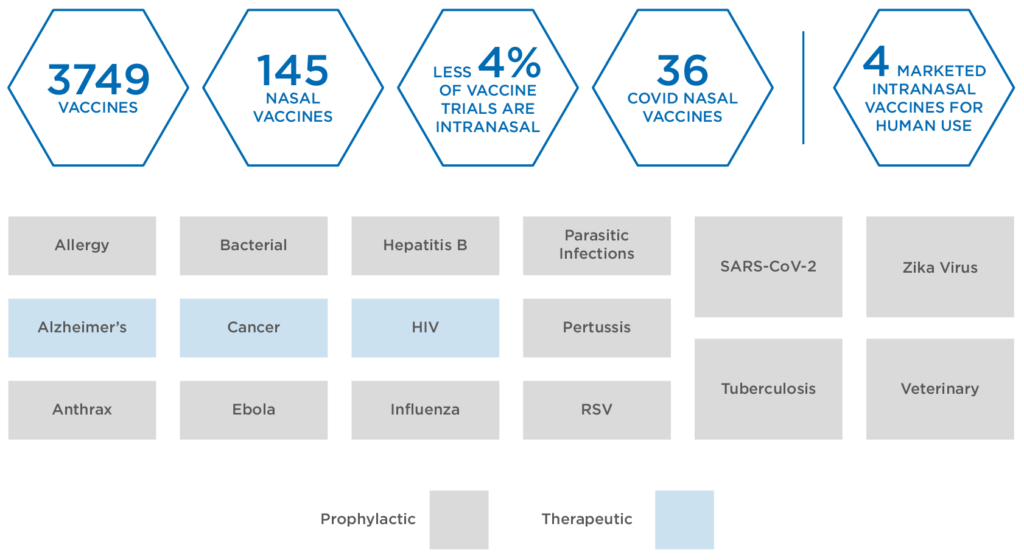
Figure 1: Global vaccines in development.
“Needle phobia is believed to affect as much as 10% of the UK population, and research has shown that it may be responsible for 10% of covid-vaccine hesitancy in the country.”
There are many factors driving this interest, but the predominant reason is that nasal vaccines can directly instigate mucosal immunity at the site of infection. In the case of SARS-CoV-2, after binding with ACE2 receptors in the nasal cavity, the virus starts to replicate before it transfers to the lungs, where the infection takes hold. Mucosal immunity would provide the first line of defence at the mucous membranes, adding another layer of protection to the systemic immunity offered by existing covid-19 vaccines. Further analysis in this emerging area is required, but it is believed that inducing mucosal immunity in this way may yield benefits not seen with conventional parenteral routes of vaccine administration.4
INTRANASAL VACCINE DELIVERY
Intranasal delivery of vaccines remains relatively uncharted territory. To date, only three intranasal products have been granted market licences for human use, including a seasonal influenza vaccine FluMist® (AstraZeneca, Cambridge, UK). Currently, exploratory work continues on 146 nasal vaccines, accounting for less than 4.4% of all active vaccine trials – of these, 40 are targeted at covid-19.
Despite this activity around intranasal delivery, patients are most familiar with vaccines being delivered by injection or oral administration. Given the breadth of vaccine treatments that employ these methods, there is a much greater depth of knowledge regarding formulation development and the associated pathways for regulatory approval. However, these methods also suffer from several challenges.
The invasive nature of injections, whether intramuscular, subcutaneous or intravenous, is an obstacle for some patients. Some will simply find the experience unpleasant, while others will find it distressing – and may actively avoid vaccination altogether where injections are required as a result. Indeed, needle phobia is believed to affect as much as 10% of the UK population5 and research has shown that it may be responsible for 10% of covid-vaccine hesitancy in the country.6 It is not only patients who are affected; healthcare professionals may also be uncomfortable due to the potential for needlestick injuries.
“In bringing an intranasal vaccine to market, challenges such as those discussed in this article underline the importance of understanding nasal physiology and vaccine formulation to elicit the desired immune response. This has been amplified in the context of covid-19, where there remains a clear urgency around developing treatments.”
These risks are eliminated with orally delivered vaccines, which can present in various forms – such as drops in the case of oral poliovirus vaccines or capsules in the case of the Typhoid fever vaccine Vivotif (Typhoid Vaccine Live Oral Ty21a) (Janssen Vaccines, formerly Crucell, Leiden, Netherlands). Oral delivery forms can also induce a mucosal, as well as systemic, immune response. However, the harsh environment of the gastro-intestinal (GI) tract, with its high acidity and presence of molecule-degrading enzymes, is known to compromise the local bioavailability of vaccines. This introduces the requirement for higher doses which, in turn, brings challenges relating to formulation development.7
Intranasal delivery similarly induces a mucosal and systemic response, but eradicates the complications associated with enzymatic metabolism in the GI tract. Using this method, drug molecules are aerosolised for deposition in the nasal cavity. For vaccines specifically, the target area is the nasopharynx-associated lymphoid tissue, an area rich in various cell types, including the immunocompetent antigen-presenting cells, such as T cells, B cells, M cells and dendritic cells, which are critical in generating local and systemic immune responses.
In terms of its anatomical and physiological structure, the highly vascularised lateral wall of the nasal cavity provides a welcome environment for drug molecule absorption. It contains immunocompetent cells, such as dendritic cells, that facilitate antigen interaction. Slightly acidic and isotonic, it has a total surface area of approximately 150 cm2, around 85% of which is covered by the respiratory region.8
THE CHALLENGES AND SOLUTIONS FOR NASAL VACCINE DELIVERY
The intranasal delivery route is not free from its own challenges, however. Many of these challenges can be attributed to the natural defence mechanisms designed to keep invasive particles, allergens, viruses and bacteria from entering the body through the nose. This includes the enzymatic processes present within the nasal cavity, which increases the likelihood of degradation and destabilisation of particulate drug compounds. Although the impact of enzymes such as cytochrome P450 is felt to a much lesser extent in the nasal cavity compared with the GI tract.
Mucociliary clearance is another important consideration when it comes to intranasal drug delivery. Although it is an incredibly helpful defence mechanism, clearance of the mucous layer lining and the nasal epithelium also obstructs the uptake of vaccines – the level of immunity achievable is directly related to the level of exposure that antigens can achieve prior to clearance taking place. Studies have determined that the normal range for nasal mucociliary clearance is up to 20 minutes, providing a clear indication of the time limitations involved.9 The residence time needed for a vaccine is currently unknown.
Mucociliary clearance can be addressed, however, by the inclusion of a series of excipients within the formulation to assist with the muco-adhesion of antigens. This underlines the importance of vaccine formulations being optimised for nasal delivery through the accommodation of the antigen itself – whether whole virus, viral vector, protein subunit or nucleic acid (RNA or DNA). In addition, adjuvants increase the potentiation and longevity of the immune response, and stabilisers maintain the integrity of the formulation and its active ingredients over time. FluMist, for example, is a suspension of live attenuated virus that incorporates gelatine, sucrose and amino acids as stabilisers, as well as excipients that control for viscosity and acidity.
In some cases, the vaccine is more stable in a non-aqueous environment. Further stabilisation can be introduced through the lyophilisation of the formulation. Here, a bulking agent may be required to help with aerosolisation of the resulting vaccine powder, which can be delivered in a higher payload, as well as avoid the need for cold chain storage as it demonstrates greater stability at room temperature. Indeed, studies of a recombinant protective antigen intranasal vaccine for anthrax indicated that effectiveness was retained at room temperature after two years.10
Particulate systems for intranasally delivered vaccines include liposomal formulations and polymeric nanoparticle formulations. Because respiratory viruses are in the range of 10–200 nm, this should also be reflected in the size of formulation particles, with a particle size of >10 μm deemed necessary to minimise deposition in the lung and a particle size of 200–300 nm deemed optimal for uptake by dendritic cells.11
As well as size, other particle characteristics can influence the level of immune response. This includes addressing the risk of particle agglomeration through the inclusion of stabilisers, and exploiting the benefit of positively charged particles, which are found to enhance interaction at the cell membrane. Indeed, cationic carrier particles, such as chitosan, a biodegradable, biocompatible polymer with mucoadhesive properties, have been found to help with vaccine uptake into the cell and the slowing of mucociliary clearance.12
Chitosan is one of several adjuvants explored for use in nasal vaccines with the purpose of stimulating and enhancing the immune response, potentially reducing the level of antigen required. In injectable vaccines, adjuvants such as aluminium salts commonly fulfil this role, but they are not suitable for nasal vaccine formulations. An example of an approved nasal vaccine adjuvant is E. coli heat-labile toxin, although it was subsequently withdrawn following concerns over adverse effects and the possibility of transportation to the brain via the olfactory neurons. As such, there are currently no widely approved adjuvants for nasal vaccines, and the avoidance of olfactory deposition continues to be a key focus in product development.
In bringing an intranasal vaccine to market, challenges such as those discussed in this article underline the importance of understanding nasal physiology and vaccine formulation to elicit the desired immune response. This has been amplified in the context of covid-19, where there remains a clear urgency around developing treatments. The US FDA’s Emergency Use Authorization provides an accelerated pathway to answer this, although regulators must be satisfied with the available data on the “chemistry, manufacturing, and controls” for vaccine candidates.13
Aptar Pharma supports pharmaceutical partners with an end-to-end service that encompasses all aspects of development and delivery for nasal vaccines, as well as providing a framework for regulatory compliance. This begins at the preclinical phase, with in vitro modelling to support target deposition studies to assess stabilisation, extending to scale-up support for GMP market-ready production.
A crucial aspect of Aptar Pharma’s multi-faceted development process is ensuring that vaccine formulations are complemented by the device to support intranasal administration. Given the prevalence of standard-adjacent containers, including blow-fill-seal, syringes and vials, flexibility and adaptability are key factors to facilitate delivery, whether for liquid or powder formulations on either single- or multiple-dose vaccines.
Aptar Pharma’s BiVaX, for example, is a bi-dose device containing an adapter that allows the syringe to be filled from a vial. The nozzle is then refixed to the device and the plunger is depressed to deliver the first dose. Turning the plunger, which acts as an integrated dose divider, allows the second dose to be delivered.
Another example is Aptar Pharma’s CPS Multidose, which enables a CPS pump to be fitted directly to the top of a standard glass container. After priming and fitting with a mass vaccination cap, the device can be used to spray the required dose into the patient’s nose. After removing the cap and disinfecting the actuator nozzle tip, a new cap can be applied to facilitate administration to another recipient, without priming.
In each case, delivery of the vaccine via the nasal cavity presents a preferable option for patients when compared with legacy injectable systems for all the reasons previously discussed. These devices and techniques are also intuitive for healthcare providers, providing a simple delivery method in both clinical and commercial settings that complements the existing landscape for vaccine containers.
Successfully arriving at this point and realising the promise of intranasal immunisation via such innovative delivery solutions relies on robust relationships between pharmaceutical companies and their trusted device development and delivery partners. These foundations are fundamental to derisk and accelerate the market readiness of nasal vaccine solutions, addressing the known hurdles (Figure 2) and responding to the ongoing demand for much-needed mucosal and systemic immunity in the face of the ongoing threat presented by SARS-CoV-2.
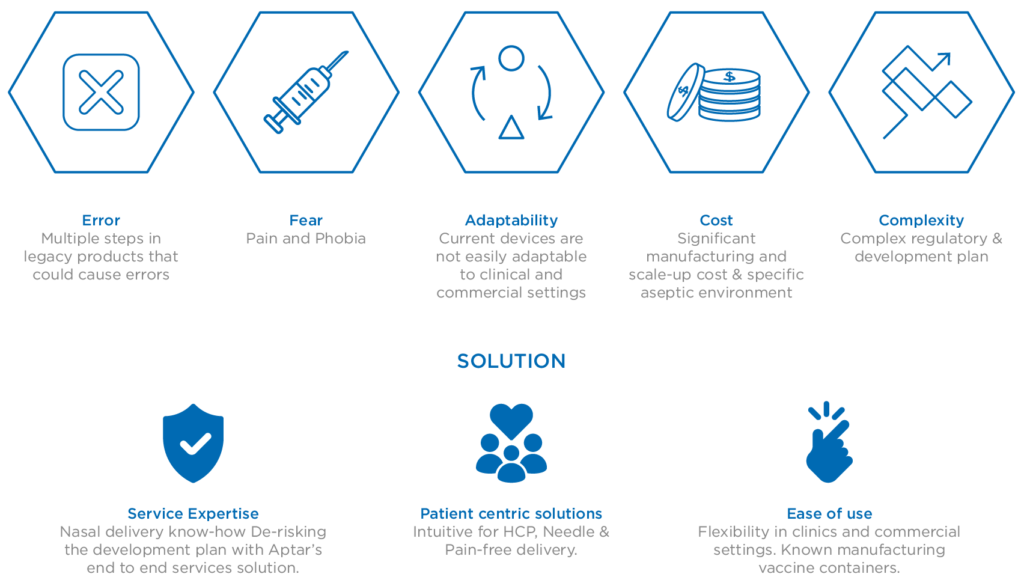
Figure 2: The challenges and solutions associated with nasal vaccine delivery.
REFERENCES
- “Coronavirus (covid-19) Vaccinations”. Our World In Data, accessed Nov 2021.
- “Global Vaccine Market Report”. World Health Organization, Working Draft – Dec 2020.
- “The covid vaccine market is worth at least $150 billion. Can we stop it being flooded with fakes?”. World Economic Forum, Jul 15, 2021.
- Russell MW, “Mucosal Immunity in covid-19: A Neglected but Critical Aspect of SARS-CoV-2 Infection”. 2020, Immunol, Nov 30, 2020.
- “Injection Phobia”. Anxiety UK, Accessed Nov 2021.
- Freeman D, “Mucosal Immunity in covid-19: A Neglected but Critical Aspect of SARS-CoV-2 Infection”. Gavi The Vaccine Alliance, Jun 17, 2021.
- Vela Ramirez JE, Sharpe LA, Peppasa NA, “Current state and challenges in developing oral vaccines”. Adv Drug Deliv Rev, 2017, Vol 15(114), pp 116–131.
- Ramvikas M et al, “Nasal Vaccine Delivery”. Micro and Nanotechnology in Vaccine Development. 2017, pp 279–301
- Deborah S, Prathibha KM, “Measurement of Nasal Mucociliary Clearance”. Clin Res Pulmonol, 2014, Vol 2(2), p 1019.
- Wang SH, “Stable dry powder formulation for nasal delivery of anthrax vaccine”. J Pharm Sci, 2012, Vol 101(1), pp 31–47.
- Hellfritzsch M, Scherliess R, “Mucosal Vaccination via the Respiratory Tract”. Pharmaceutics, 2019, Vol 11(8), p 375.
- Mohammed MA, “An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery”. Pharmaceutics. 2017, Vol 9(4), p 53.
- “Emergency Use Authorization for Vaccines Explained”. US FDA, Nov 20, 2020.

