To Issue 131
Citation: Minelli C, “An Invitation to Participate in Standardisation of Particle Measurements for Pharmaceuticals”. ONdrugDelivery, Issue 131 (Apr 2022), pp 12–15.
Caterina Minelli discusses the importance of co-operation between metrology institutes and the pharmaceutical industry in the pursuit of improving analytical techniques and standards for the characterisation of particle attributes in drug development.
Innovation in the field of analytical technology and the standards for measuring particle attributes is a continual process, driven in large part by the importance of particles in the development and manufacture of pharmaceutical formulations. Characterisation of particles is central to ensuring the efficacy of many medicinal products, including dry powder inhalers, APIs, excipients within solid oral dosages, micro-particulates and both oil and solid phases in topical formulations. Advanced therapies that use liposomal and lipid nanoparticles, along with other nano preparations, are vital to treatments including anticancer therapeutics, vaccines and vectors for gene delivery applications. Particle characterisation is integral to understanding the performance and implementation of quality control for medicines.
“The pace of innovation and the increasing complexity of particle- based products pose a substantial regulatory challenge and ensuring that the measurement infrastructure remains relevant and comprehensive is essential to enable new medicine development.”
The pace of innovation and the increasing complexity of particle-based products pose a substantial regulatory challenge and ensuring that the measurement infrastructure remains relevant and comprehensive is essential to enable new medicine development. It is not surprising, then, that the measurement technology serving the pharmaceutical industry is continuously evolving, and that standard bodies, regulators and national metrology institutes are proactive in developing the underpinning measurement infrastructure. The collaboration of such institutions with the pharmaceutical industry is essential for defining and prioritising measurement needs.
MEASURING CRITICAL ATTRIBUTES
There are many different characteristics, or attributes, of particles that can be measured. However, it is not always clear which of these attributes are critical to pharmaceutical product performance. Furthermore, the selection of which attributes should be measured may change during the lifecycle of a product, with many more attributes typically being measured in the development phase than in manufacturing control.
The type and specifications of analytical instruments employed also changes across the different stages of a product’s lifecycle. During development, a detailed mechanistic understanding of a drug’s mode of action or the performance of a formulation requires state-of-the-art measurement capabilities and expertise, which may be available in-house but is often accessed through specialist outsourcing partnerships. As a product progresses towards larger volume manufacturing, the number of measured attributes is reduced, while the quality infrastructure typically relies on analytical instrumentation that is available at the production site. Sensitivity, precision and accuracy requirements for the analytical instrumentation change in accordance with the product phase.
There is a wide variety of particle types used in therapeutic formulations and their constituent materials and no single analytical technique addresses all types of particles. Particle measurements during the product lifecycle may include physical and chemical characteristics, drug loading, release profile and particle fate in animal models, to mention just a few. Measurement methods are best used in concert to provide complementary pieces of information that, like the pieces of a jigsaw, may be combined to enable measurement experts to build a complete picture of a product. To reap the benefits of this synergistic approach, effort should be invested into ensuring that both sample preparation and measurement approaches are consistent across techniques to enable comparability.
“Knowledge of the changes that particles undergo with time and when exposed to different environments can enable a better mechanistic understanding of their mode of action.”
It is important to consider that particles are dynamic systems and that their attributes may change with time or with the environment that they are exposed to. For this reason, particle formulation measurements must be taken with the particles in both their pristine state and in either simulated or real biological environments over time. Knowledge of the changes that particles undergo with time and when exposed to different environments can enable a better mechanistic understanding of their mode of action. In a similar way, it is useful to measure particles while exposing them to varying temperature and humidity levels in order to inform understanding of potential changes to the product during storage and ageing. Both of these examples involve significant complexity from a measurement technology point of view.
One aspect of this complexity is the challenge of measuring particles in matrices, including those of a biological nature. The ability to measure particle products in complex matrices requires that either:
- The particles are separated from the matrix prior to measurement
- The analytical method can distinguish between the particle and the matrix.
To address the first, significant effort is invested in developing preparative protocols and technologies that minimise changes to particle attributes during separation. These protocols tend to be designed on a bespoke basis for each product and matrix combination and the time needed for their development and validation should not be underestimated.
Methods that can distinguish the particles from the matrix include both imaging and spectroscopy. Among the available imaging methods, optical and electron microscopies are widespread but are best coupled with chemical strategies to identify the products within the matrices. For example, fluorescence dyes conjugated with the molecules of interest afford multiplexed fluorescence imaging, including super-resolution methods. Among label-free methods, some types of Raman spectroscopy and mass spectrometry enable the location and quantification of the product ingredients within biological cells and tissues or a medical device. Raman-based and fluorescence-based microscopies are also suited to in vitro imaging where, for example, the migration of active ingredients following their release may be observed and measured.
“A robust measurement infrastructure for pharmaceutical product development and manufacturing ensures that particle attributes can be compared over time and at different production sites with confidence.”
BUILDING CONFIDENCE IN MEASUREMENTS
A robust measurement infrastructure for pharmaceutical product development and manufacturing ensures that particle attributes can be compared over time and at different production sites with confidence. This is essential for evaluating product consistency across different batches and changes due to ageing, as well as informing the decisions that will direct product improvements and diversification.
Documentary standards and reference materials underpin the confident use of analytical technology. Such standards are typically either expert reports or prescriptive protocols describing terminology, practice for sample preparation and measurement, and relevant aspects of data analysis. Importantly, documentary standards contain knowledge about the potential sources of errors in a measurement and enable the description of particle attributes in terms of values with associated uncertainties. Published standards are periodically updated to enable them to keep pace with innovations in measurement technology.
Standards result from significant expertise developed by measurement laboratories and instrument manufacturers, generally over a number of years, and are cowritten and internationally reviewed by measurement experts. Technical committees (TCs) relevant to particle metrology include the International Organisation for Standardisation (ISO) TC 229 – Nanotechnologies and TC 24 – Particle Characterisation; ASTM International E55 – Manufacture of Pharmaceutical and Biopharmaceutical Products and E56 – Nanotechnology; the European Committee for Standardisation (CEN) TC 352 – Nanotechnologies; and the British Standards Institution (BSI) LBI/37 – Particle Characterisation Including Sieving. Pharmacopoeias also contain practice and reference databases useful to particle analysis. The interactions across these technical committees are vital and it is not unusual for one expert to sit on many related committees, but harmonisation of standards could be improved through more effective co-operation between bodies.
Lists of published documentary standards are typically available on the websites of the standards bodies where they are available for purchase or through open access models. The standards body websites also often list the documentary standards currently under development; relevant examples include a document to define liposome-related terminology (ISO/AWI TS 4958), a guide to measuring nanoparticle agglomeration and aggregation (CEN/TC 352) and a guide to characterising the encapsulation, extraction and analysis of mRNA in lipid nanoparticles (ASTM WK75607).
To prepare for the development of a documentary standard, it is usual to complete one or more inter-laboratory studies or round-robin comparisons. Depending on the type of study and the nature of the organisations involved, the primary focus of the study may alter. For example, participation in the highest level of inter-laboratory comparisons organised by the International Bureau of Weights and Measures (BIPM) is restricted to designated national measurement institutes (NMIs) and the focus is on the accuracy and reproducibility of the measurement.
Organisations such as the Versailles Project on Advanced Materials and Standards (VAMAS) encourage wider participation from industry, academia and research laboratories with a primary focus on comparability in measurement results and on testing of sample preparation and measurement protocols prior to standardisation. The outcomes of BIPM studies are used to enable the participating institutes to generate approved calibration and measurement capabilities (CMCs) and to state the attributes of certified reference materials (CRMs) with a clear uncertainty statement in both cases. Both CMCs and CRMs are the highest point of reference for evaluating measurement outcome.
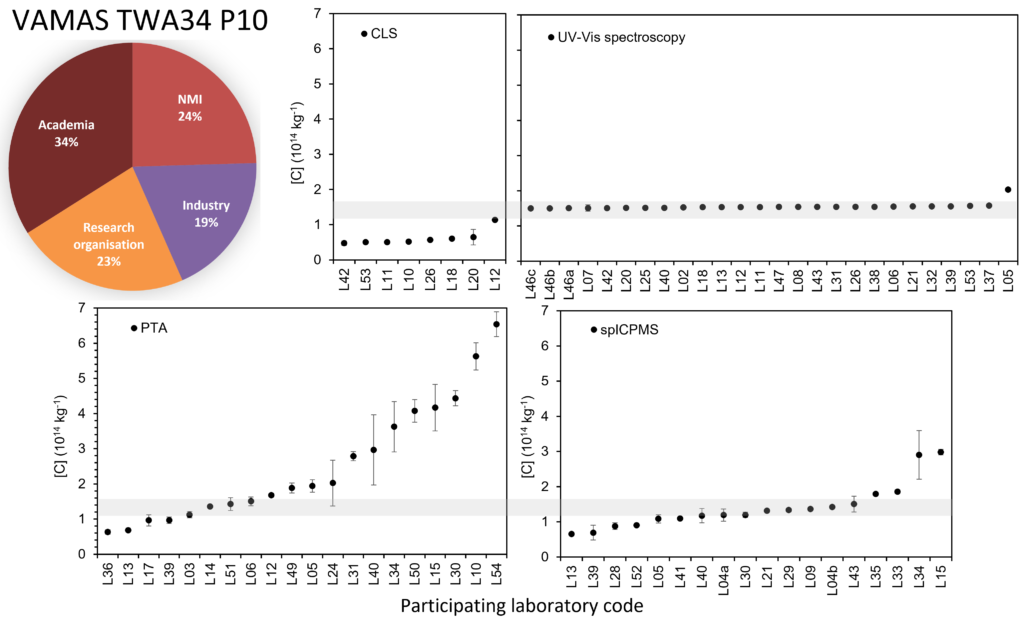
Figure 1: Outcome of the VAMAS TWA 34 Project 10 inter-laboratory comparison on the measurement of the number concentration of colloidal gold by centrifugal liquid sedimentation (CLS), UV-visible spectroscopy, particle tracking analysis (PTA) and single particle inductively coupled plasma mass spectrometry (spICPMS). The pie chart shows the background of the laboratories enrolled to the study. The pie chart shows the background of the laboratories participating in the study.
Inter-laboratory comparisons open to a broader community of participants, such as those run under VAMAS, are required to establish and disseminate best practice, underpin and test documentary standards, and benchmark participating laboratories’ measurement capability. For example, Figure 1 shows the outcome of a large VAMAS interlaboratory study organised by the National Physical Laboratory (NPL) on measuring the number concentration of colloidal gold.1 The study provided objective data to compare practice and performance associated with five independent methods and to cross-validate independent measurement approaches, which is important for regulatory purposes.
The study clearly highlighted that some methods have very small between-lab variability, suggesting them as methods of choice for quality control purposes. However, where accuracy is required and for other types of particles, the choice may be different. Not all methods are suitable for all materials and particle size ranges; some materials may become unstable upon dilution or samples may be expensive and limited in volume, which imposes different methodological requirements. The outcomes from this study are currently being incorporated into a draft ISO technical report (ISO/DTR 24672) scheduled for publication in late 2022. Through this ISO document, best practice for particle number concentration measurement will be available to the entire community.
An important aspect of a VAMAS study is that the publication of its datasets, combined with the commercial availability of the test materials, offers a straightforward route for laboratories to benchmark and demonstrate the performance of their measurement capability. Confidence in measurement capability enables faster development and more robust quality frameworks and product regulatory submissions. For this reason, there is a need to extend inter-laboratory comparisons to a broader selection of particles that mimic the attributes of real particle-based products more closely, and which are dispersed or distributed in relevant matrices.
INVITATION TO COLLABORATE
ISO/DTR 24672 is just one of the many efforts that the particle metrology community is engaged in with the intention of continuing to develop a robust measurement framework to underpin product development, manufacturing and regulatory approval. There is a need to maintain such frameworks’ relevance and fitness for purpose. This is a significant challenge considering the fast pace of innovation in pharmaceutical products and the fact that documentary standards are usually developed over several years.
There is a need to grow the channels of communications between the pharmaceutical industry, the metrology community and regulators to inform the discussion on product innovation and standardisation needs. For example, particle-based products are increasing in complexity, becoming more structured and sophisticated in the way they deliver their therapeutic cargo while improving their safety profile. Efforts to develop a robust metrology framework in biology are underway but are relatively new, with more work needed to fully standardise measurement methods supporting in vitro and in vivo testing.2 Finally, the step change promised by the digitisation of the industry requires robust documentary standards that establish a common vocabulary and enable the implementation of findable, accessible, interoperable and reusable (FAIR) data principles.
Stronger collaboration between NMIs, industry, governments and regulators will accelerate the pace of innovation and the commercialisation of particle-based products, from paediatric medicines to new vaccines. Initiatives such as inter-laboratory studies are an excellent vehicle for collaboration. There is a need for the industry and measurement development scientists to engage at early stages of product development to identify challenges, understand the complexities involved and start developing the next generation of analytics capable of tackling such obstacles to innovation. There are some excellent examples of funding schemes made available by governments around the world to foster this co-operation, but more strategic routes to embed the work of NMIs within industrial innovation must be opened. Finally, the pharmaceutical industry and regulators play a critical role in determining measurement priorities, directing the metrology community to develop methods, standards and reference materials that can accelerate the delivery of improved therapeutics and ultimately bring improved outcomes to patients.
REFERENCES
- Minelli C et al “Versailles Project on Advanced Materials and Standards (VAMAS) Interlaboratory Study on Measuring the Number Concentration of Colloidal Gold Nanoparticles”. Nanoscale, Mar 2022, epub.
- Sené M, Gilmore I, Janssen JT, “Metrology is Key to Reproducing Results”. Nature, Jul 2017, Vol 547(7664), pp 397–399.

