To Issue 131
Citation: Klein M-C, Bilstein A, Kraus R, “Nose-to-Brain Drug Administration and a Novel Approach with an Old Familiar”. ONdrugDelivery, Issue 131 (Apr 2022), pp 22–24.
Marie-Christine Klein, Andreas Bilstein and Rouven Kraus look at the advantages of the nose-to-brain route and soft mist nasal sprays for drug administration.
Bestselling intranasal drugs mostly cover the locally acting symptoms of colds and allergies. However, nasal drug delivery opens up a sophisticated plethora of possibilities, based on the anatomical advantages of the nasal cavity and its close proximity to the blood-brain barrier (BBB) – the brain protector and stressor of drug administration.
In contrast to other organs where a vivid exchange of molecules between blood and organs exists, the brain tissue is separated from the blood by the BBB, which consists of different cell types that fuse to an extremely tight barrier. The BBB’s physiology is such that only very small, lipophilic molecules, or molecules with their own specialised transport systems in the brain epithelium, can overcome it. This means that, on one hand, the BBB can be viewed as an evolutionary marvel, effective at safeguarding the brain against pathogens and toxins and creating a highly specialised environment. But on the other hand, from the point of view of pharmaceutical therapeutics, the BBB can be seen as a barrier in the negative sense, hindering effective drug targeting of brain-associated disorders of the central nervous system (CNS). To open the BBB pharmacologically to facilitate drug uptake is both difficult and dangerous because it is always accompanied by the danger of an ingress of toxic plasma proteins and, as a consequence, neurotherapeutics. Sometimes, drug design is able to adapt or exploit these special circumstances, but most of the potential neurotherapeutics (roughly 98%) will not be able to reach their intended target.1
“Another advantage of the intranasal route consists of overcoming the hepatic first-pass effect that results in higher bioavailability of the drug, in a lower dosing and, consequently, in fewer side effects.”
ANATOMY OF THE OLFACTORY REGION
One possibility to bypass the BBB and enter the brain is drug administration via nerval structures that invade the brain, the so-called trigeminal and olfactory pathways. Zooming into the anatomy of the upper respiratory tract, the close connection of the brain and the nasal cavity becomes obvious.
The nasal cavity and its mucosa are essential for passaging and purifying inhaled air. The very anterior region of the nose, the so-called vestibular region, has a surface of ~0.6 cm2, and its main mission is to filter inhaled particles with the nasal hairs.2 The prefiltered air flows further along the cavity in the respiratory region. This region, covering the majority of the nasal cavity with a volume of ~130 cm2, is highly vascularised tissue.2 That means many small blood vessels innervate the epithelium underneath and enable an extensive exchange of molecules, which is also important for systemic administration of drugs.3 The respiratory region has interfaces with branches of the trigeminal nerves that originate in the brain and provide a direct connection to the CNS.4
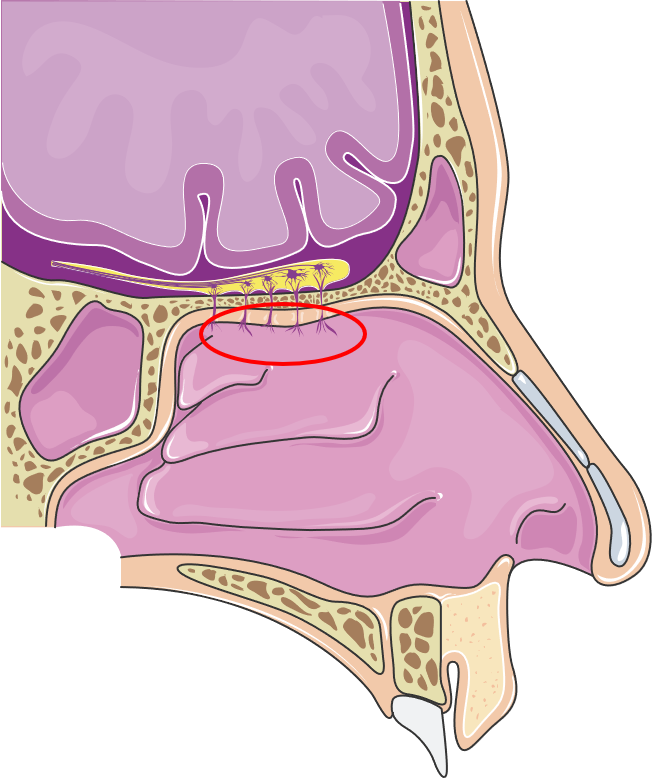
Figure 1: Anatomy of the nasal cavity emphasising the olfactory region. (Image licensed under CC BY 3.0; smart.servier.com, 16.03.2022.)
In the respiratory region, intruding particles such as dust or bacteria are trapped by the mucus and transported towards the throat. This is facilitated by various cells producing and transporting mucus, resulting in mucociliary clearance. Further, molecules that enter the nasal cavity are dissolved in the mucus and transported to a specialised region in the nasal cavity, the olfactory region. It lies in the very roof of the cavity (Figure 1) where the olfactory mucosa has a unique structure. It is interspersed with olfactory neurons that access the molecules that are transported to the olfactory region in fluid film – a perfect environment for odorous molecules. The nervous anchors of the neurons congregate to form the olfactory nerve and provide a direct connection from the nose to the brain. Simplified, molecules that enter the nose are translated in the brain into the sense of olfaction.
NOSE-TO-BRAIN ROUTE OF DRUG ADMINISTRATION: CHANCES AND LIMITATIONS
The nose-to-brain route of drug administration combines a lot of advantages. The possibility of bypassing the BBB opens up a noteworthy research area for the treatment of neurodegenerative diseases for which promising data already exist, e.g. on Parkinson’s disease5 or intranasally administered insulin for memory loss.6 The very common and established use of nasal sprays or drops supports self-administration and, at the same time, good treatment adherence. Moreover, the risk of infection is diminished and a vast number of side effects from invasive methods, such as intracerebroventricular administration, which is used to apply chemotherapeutics to circumvent the BBB, are eliminated.
Another advantage of the intranasal route consists of overcoming the hepatic first-pass effect that results in higher bioavailability of the drug, in a lower dosing and, consequently, in fewer side effects. The limitations of the nose-to-brain target route are mainly the anatomically given small volumes (25–200 μL) that can be applied to the nasal cavity, which may limit the use for potent drugs. Moreover, the driving force of the ciliated cells of the respiratory cavity towards the pharynx, the so-called mucociliary clearance, limits the drug interaction time with the epithelium and is also influenced by the chemical nature of the drug molecule and its formulation. Chemical properties, such as the polarity, the pH value and the viscosity, play a crucial role in drug formulation for intranasal applications.
A major task for future research in this area is to formulate drugs that can overcome the limitations of intranasal administration in general and the nose-to-brain target route specifically. Key to this will be an adequate and formulation-compatible delivery system that allows a targeting of the olfactory region within the nasal cavity.
“Using a pump that unites the short actuation time and easy handling of a standard spray pump, and a distribution pattern that resembles a nebulising system applied over several minutes,10 is a winning alternative.”
DELIVERY OF DRUGS TO THE OLFACTORY REGION
For effective drug uptake by the nose-to-brain pathway, the formulation is dependent on both the chemical and biological ability to permeate the epithelium and drug deposition in the olfactory region. To derive characteristics that allow an approximated prediction of effective targeting, some technical performance factors influencing the deposition need to be taken into consideration. These characteristics would be the droplet size distribution, aerodynamics, the plume geometry and the spray pattern of the administered nasal drugs.
Data from laser diffraction allow the evaluation of the spray distribution generated by the applicator system of choice. If small droplets <5 μm are generated, they will not usually stay in the nasal cavity but will find their way to the lower respiratory tract. The higher the droplet size, the more anterior parts of the nasal cavity will be targeted. It is not clear what is the best droplet size to reach the olfactory region but there are suggestions that a diameter of ~10 μm may be favourable.7
Standard nasal sprays, which are commonly used for treating local pathologies such as nasal constriction and allergy symptoms, mainly have a spraying profile that generates droplets >50 μm that are deposited with a high velocity. To target the olfactory region with its small surface fraction of the total cavity (5.2 %),8 a well-balanced droplet size must be created that ensures the deposition mainly at the entry of the nose and, at the same time, excludes deposition at the very anterior part to prevent a quick removal.9
An easily accessible model to gain the global information on spray deposition in the whole respiratory tract is the Koken® nasal cast (Koken Co, Toyko, Japan) model. Although limited by being based on the anatomy of one individuum, it allows the deduction of the deposition profile of a formulation applied to the model. Allocating the cast in different fractions representing, for example, the olfactory region, the vestibule and the turbinates may be an advantage in fine tuning the formulation in the respective application system and provide a winning tool in the formulation development.
The strong correlation of the deposition profile in the upper respiratory tract and aerodynamics suggests that liquid nebulising could be an optimised means of targeting the olfactory region by combining low velocity and smaller droplet sizes.10
SOFT MIST NASAL SPRAY AS A NEW TOOL FOR DRUG ADMINISTRATION
Established nasal spray pumps are limited in their ability to fulfil the described drug delivery needs. Therefore, using a pump that unites the short actuation time and easy handling of a standard spray pump, and a distribution pattern that resembles a nebulising system applied over several minutes,10 is a winning alternative. Together with Medspray, a soft mist nasal spray pump has been developed that combines the nebulisation technology with an easy, effective and well-known application system, a 3K® metered dose nasal spray pump. A set of pilot data is depicted in Figure 2 and compares different pumps in their distribution pattern of an aqueous solution with a fluorescence dye. The evenly spread spray mist of the 3K® UltraSoft pump reaches every compartment of the nasal cavity, including the olfactory region. This is an outstanding result, which may result from the long actuation time (~1 sec) in combination with small droplet size distribution of the spray pump compared with the other pumps under examination.
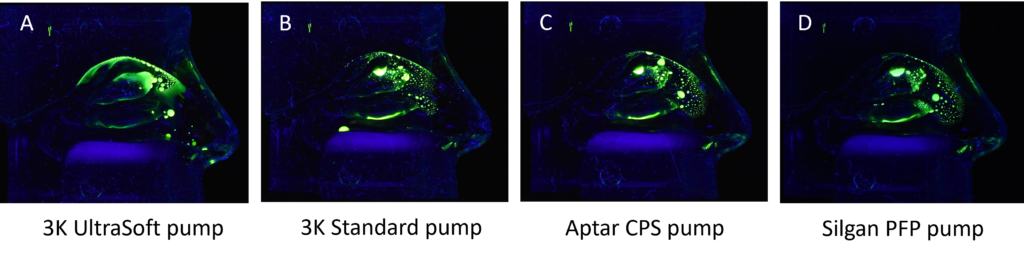
Figure 2: Comparison of different pumps concerning their distribution pattern of an aqueous solution in a Koken® nasal cast under continuous air flow of 15 L/min.
Since the intranasal application is highly dependent on both anatomical differences of the nasal cavity between individuals and their actuation behaviour, two different spray angles were tested regarding their ability to target the olfactory region. The results are shown in Figure 3.
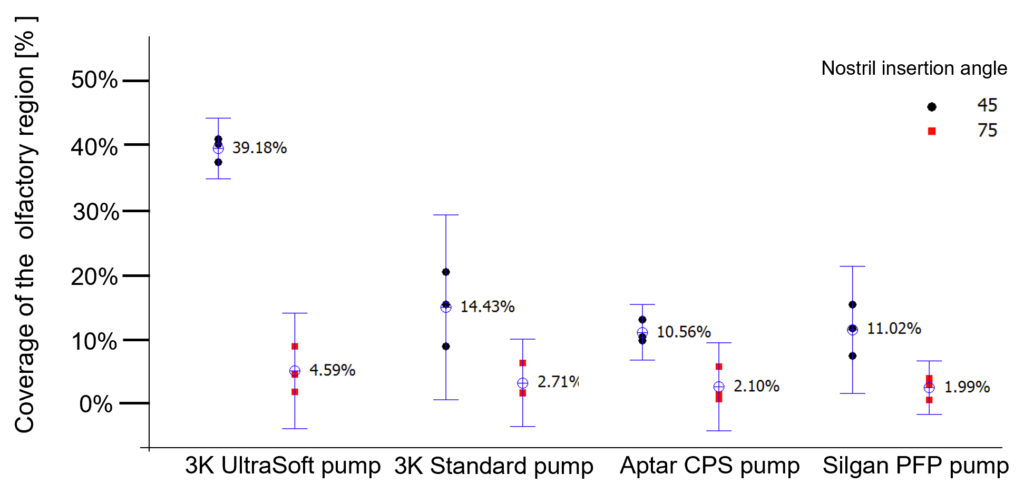
Figure 3: Influence of different spray angles and different pump systems in the coverage of the olfactory system by quantification of an aqueous solution distributed in a Koken® nasal cast.
Compared with the other spray pumps tested, the standard 3K® pump achieved the best coverage of the olfactory region (14%).A further remarkable increase and optimised coverage of ~40% of the olfactory region was achieved by the soft mist pump when the spray head was inserted into the nostril with an insertion angle of 45°. This is an astonishing result considering that the olfactory region represents only ~5% surface fraction of the total nasal cavity.
For future research, it is reasonable to assume that by using the soft mist spray pump and generating an evenly spread distribution pattern over the whole respiratory region, a positive effect on the intranasal systemic target can also be achieved, as the velocity and droplet size are ideal to overcome the anatomical and application constraints due to the slow application and reduced spray force with less impaction.
CONCLUSION
The nose-to-brain route of drug administration via nasal spray combines some powerful advantages of drug availability, being a fast-onset treatment solution with high bioavailability, the potential to reduce side effects and self-administration with a perfectly easy and safe usability profile.
In contrast to explanation-intensive and costly systems dedicated to a nose-to-brain application, the soft mist generating spray pump could be a solution for nose-to-brain applications and exert an advantageous impact on the therapeutic possibility of brain-associated diseases. Moreover, it could also be an uncomplicated added value for a plethora of indications and may also generate better systemic uptake of existing drugs. Further, the ability to combine the soft mist pump with either a preservative-free or a preserved pump set-up results in a versatile application spectrum.
REFERENCES
- Pardridge W, “The Blood-Brain Barrier: Bottleneck in Brain Drug Development”. NeuroRx, 2005, Vol 2, pp 3–14.
- Erdo F et al, “Evaluation of intranasal delivery route of drug administration for brain targeting”. Brain Res Bull, 2018, Vol 143, pp 155–170.
- Arora P, Sharma S, Garget S, “Permeability issues in nasal drug delivery”. Drug Discov Today, 2002, Vol 7(18), pp 967–975.
- Crowe TP et al, “Mechanism of intranasal drug delivery directly to the brain”. Life Sci., 2018, Vol 195, pp 44–52.
- Sekerdag E et al, “A potential non-invasive glioblastoma treatment: Nose-to-brain delivery of farnesylthiosalicylic acid incorporated hybrid nanoparticles”. J Control Release, 2017, Vol 261, pp 187–198
- Born J et al, “Sniffing neuropeptides: a transnasal approach to the human brain.” Nat Neurosci, 2002, Vol 5(6), pp 514–516.
- Schroeter J et al, “Experimental measurements and computational predictions of regional particle deposition in a sectional nasal model”. J Aerosol Med Pulm Drug Deliv, 2015, 28(1), pp 20–29.
- Xi J et al, “Visualization and Quantification of Nasal and Olfactory Deposition in a Sectional Adult Nasal Airway Cast”. Pharm. Res., 2016, Vol 33, pp 1527–1541.
- Salade L et al, “How to characterize a nasal product. The state of the art of in vitro and ex vivo specific methods”. Int J Pharm, 2019, Vol 561, pp 47–65.
- Kundoor V and Dalby R, “Assessment of Nasal Spray Deposition Pattern in a Silicone Human Nose Model Using a Color-Based Method”. Pharm Res, 2010, Vol 27(1), pp 30–36.

