To Issue 134
Citation: Williams C, Kobashi N, Weber N, “Meeting an Unmet Pharma Need: Pioneering the Way to a Connected Future”. ONdrugDelivery, Issue 134 (Jun 2022), pp 55–60.
Chelsea Williams, Nagisa Kobashi and Nils Weber discuss the digital transformation of injectables, where drug formulation and device are enmeshed within a connected ecosystem powered by patient data.
“Digital disruption and the potential for scaling concepts at speed must also align with the fundamental principles that underpin patient care.”
Covid-19 has proven to be a catalyst for significant change across many industries, and there can certainly be no doubt of the influence it has had on accelerating the digital future of healthcare.
The confluence triggered by the pandemic presented an opportunity for accelerated change, with long-discussed innovations in healthcare delivery quickly embedding themselves as part of the everyday patient experience. An example is the dramatic shift towards the use of telehealth – now a prevalent patient-clinician interface, having spiked during the pandemic at a use level that was a remarkable 78 times higher than the pre-pandemic period for office visits and outpatient care.1
But while the industry can celebrate such step-change developments, there remains some distance to go before it can be argued that healthcare’s digital transformation is complete. There are many reasons behind this, of course, but culture arguably plays its part. Reid Hoffman, the co-founder of professional networking platform
LinkedIn, famously pointed out that “Silicon Valley is a mindset, not a location” in reference to the fast-moving, disruptive, entrepreneurial culture present among many firms based in the technology sector’s spiritual heartland.
In healthcare, there is certainly evidence of an appetite for the innovation this culture can unlock, but it is understandably tempered by an overarching responsibility for patient safety and improved health outcomes. Digital disruption and the potential for scaling concepts at speed must also align with the fundamental principles that underpin patient care. The stakes for adopting a Google-style “fail-fast” philosophy are simply too high.
SUPPORTING PHARMA ON THE ROAD TO INNOVATION AND INTEGRATION
Pharmaceutical companies can therefore find themselves in a situation where a digitally enabled future is coming ever more clearly into view, but they do not necessarily have an obvious path to get there. Connected healthcare is evolving fast and has great potential to answer unmet patient needs, but drug manufacturers themselves have unmet needs in terms of how to realise all the possibilities that continue to emerge.
SHL Medical acts as a guide for pharma partners facing such challenges. With a collaborative, innovative culture that has brought more than two decades of leadership in injectable drug-delivery devices, it ispioneering new digital healthcare pathways that will result in the creation of remotely managed, patient-empowered, data-rich platforms for the continuous care of patients with chronic conditions. The company’s role as an enabler for pharma partners means it is focused on mapping therapies with connected devices as part of a digital ecosystem. Its offering is flexible by design, both of its ability to engage at any level desired by its partners and in its ability to integrate with third-party patient interfaces and systems for data capture and processing.
Digital ecosystems are characterised typically as a synergistic network of digital communities functioning as a unit and linked through the flow of information across stakeholders, institutions and digital devices.2 In turn, Dr Gloria Iyawa et al noted that incorporating healthcare into the emerging digital network would create a digital health innovation ecosystem (DHIE), as seen in Figure 1, which incorporates components from care service delivery, digital health, innovation and digital ecosystems in administering patient-centric services.3
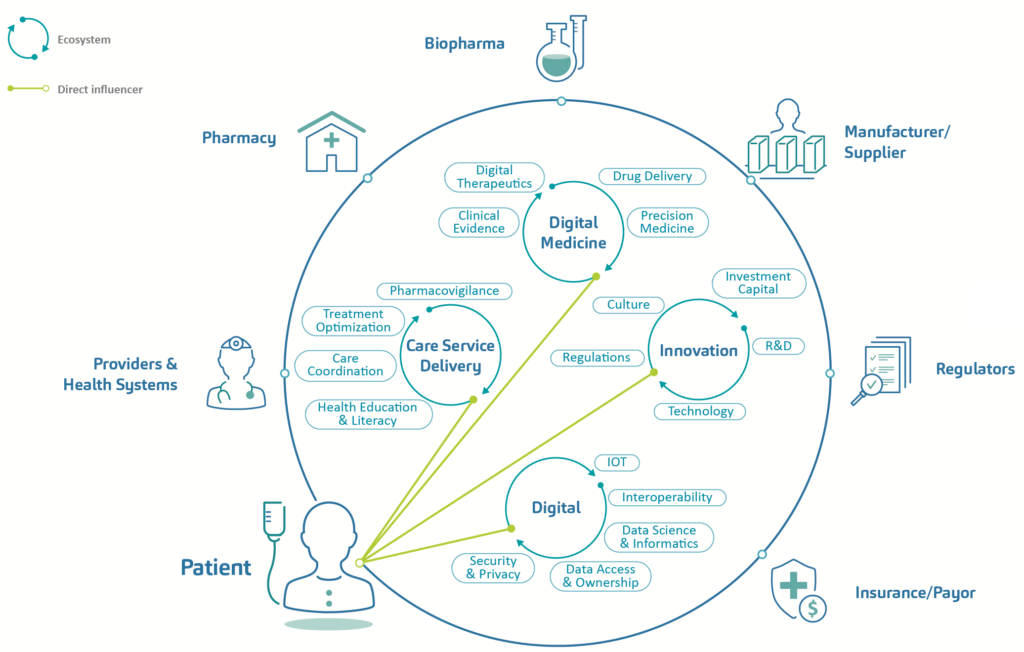
Figure 1: Components of a patient-centric digital health innovation ecosystem.
Patient centricity is a key element in the drive towards digital health. With an increasing emphasis on self-management of conditions and shared decision making between healthcare providers (HCPs) and patients, such a model for digital healthcare lays the groundwork for patient-focused systems predicated on personalised, precise and co-ordinated care delivery. Applied appropriately, technology is enabling the delivery paradigm to shift away from providers and institutions and towards healthcare recipients and their home or care setting, facilitating the data capture that enables the treatment process to be augmented by ongoing monitoring and even predictive and preventative care. As patients embrace digital tools and services as key enablers in allowing them to become gatekeepers of their own health, this will lead to increased speed of technology adoption and, in turn, economies of scale.
“Patient centricity is a key element in the drive towards digital health.”
PUTTING THE PATIENT AT THE CENTRE
While still in its infancy, the DHIE model has significant potential, and it is clear that the complexity involved will demand deep-rooted partnerships between digital device innovators, biopharmaceutical companies and healthcare providers to trigger exponential growth. Co-ordination, intent, engagement and collaboration are essential between stakeholders – and the broader the cohort of partnerships, the greater the likelihood of potential success.
SHL Medical is ideally positioned to leverage key competencies in line with potential partners, offering the right skills, technology assets and strategic vision to be a leader in healthcare delivery solutions, chiefly for injectable drug delivery. The company believes digital healthcare solutions must be friction-free at the point of use while also being aligned and integrated throughout the treatment chain. All stakeholders, from patients to HCPs and potentially payers, must be co-ordinated at strategic and operational levels to ensure the “oil” in the system – data – flows through the appropriate channels from its original source, the patient.
Medical device companies have previously approached digital health and digital transformation from a top-down, product-first perspective, akin to the “if you build it they will come” philosophy espoused in the film Field of Dreams. However, this resulted in a focus on the development of bespoke solutions with no guaranteed market or infrastructure to sustain the innovation and create economic value. The industry has since re-evaluated its strategies, instead favouring a bottom-up approach that starts with the patient – identifying their real needs and building solutions from that point of certainty.
This patient-centric approach to the design of new devices can be seen in the increased emphasis on digital strategies and investments by pharma companies, which are placing renewed attention on digitalising their portfolios.4 Several analogue devices have graduated into the self-managed digital space to address unmet patient needs, including, for example, activity trackers, titration devices and even inhalation drug-delivery devices. In the case of connected self-injecting drug-delivery systems, innovation has the potential to enhance home-based treatment, reach unserved patient populations and support patients with adherence. Unlike other digital health technologies, however, the autoinjectable market has yet to determine the best approaches and avenues to provide meaningful and actionable data to patients.
A framework for next-generation connected autoinjectors, shown in Figure 2, demonstrates the stages of technological transition from a simple device to connected therapeutic.3 The ultimate stage in this evolution refers to the augmentation of biologic molecules through sensors and connectivity, exploiting the accessibility, personalisation and data outcomes associated with a digital therapeutic. Here, there is seamless measurement of the patient experience with increased levels of fidelity and feedback. But reaching this endpoint on the digital transformation pathway is predicated on securing the incremental steps that precede it, namely the development of the connected device and, subsequently, the integrated device. This approach – of setting and achieving realistic stepped goals within a transparent structure – supports the continuous improvement necessary for maintaining momentum and limiting development risk.
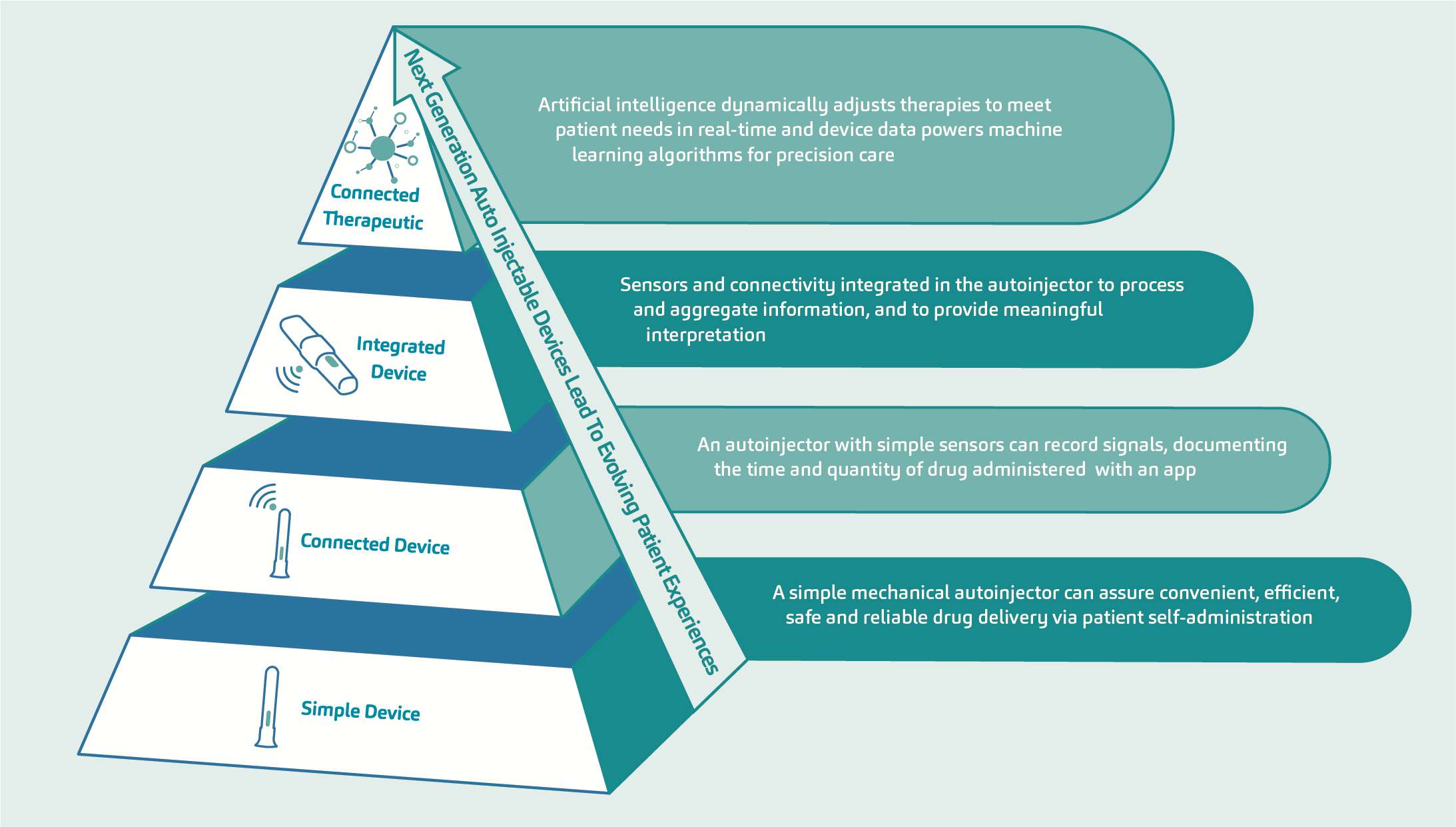
Figure 2: Cumulative value generation and implications of device complexity – evolution of the patient experience.
SHL Medical envisions the future of autoinjectors to have multiple components that will facilitate the harvesting of more – and more objective – data for use by patients, healthcare providers and payers. With each step, the technological capabilities of an autoinjector are expanded, graduating from simple adherence tracking via an add-on sensor to a feedback-loop device that can influence direct therapeutic management by the HCP. The ultimate step on this pathway is the evolution of an autonomous closed-loop system employing advanced data analytics and artificial intelligence (AI), such as those currently being explored in the diabetes space in relation to the artificial pancreas.
“The autoinjector has the potential to become the focal point of decentralised precision care for many chronic conditions, powering AI disease-management systems that impact overall patient care.”
Through this design evolution, the autoinjector has the potential to become the focal point of decentralised precision care for many chronic conditions, powering AI disease-management systems that impact overall patient care.4 It can inform a shift in the relationship dynamic between patients who are given greater autonomy and responsibility for their own healthcare and physicians overseeing home-led treatment predominantly from a remote position.
A PARTNERSHIP APPROACH BUILT ON FLEXIBILITY AND INTEGRATION
Successfully transitioning to a co-development model depends highly on the device-pharma partnership, which should be established on the basis of co-creation and collaboration. SHL Medical has conceptualised the innovation partnership shown in Figure 3, which leverages SHL Medical’s technological capabilities with pharma’s clinical innovations. This adaptable framework builds on the foundations of the verification, analytical validation and clinical validation (V3) approach for determining fit-for-purpose biometric monitoring technologies.5 Crucially, it enables the co-development of innovation partnership solutions in alignment with pharma objectives, but also in a way that keeps risks relatively low as they extend into the digital space.
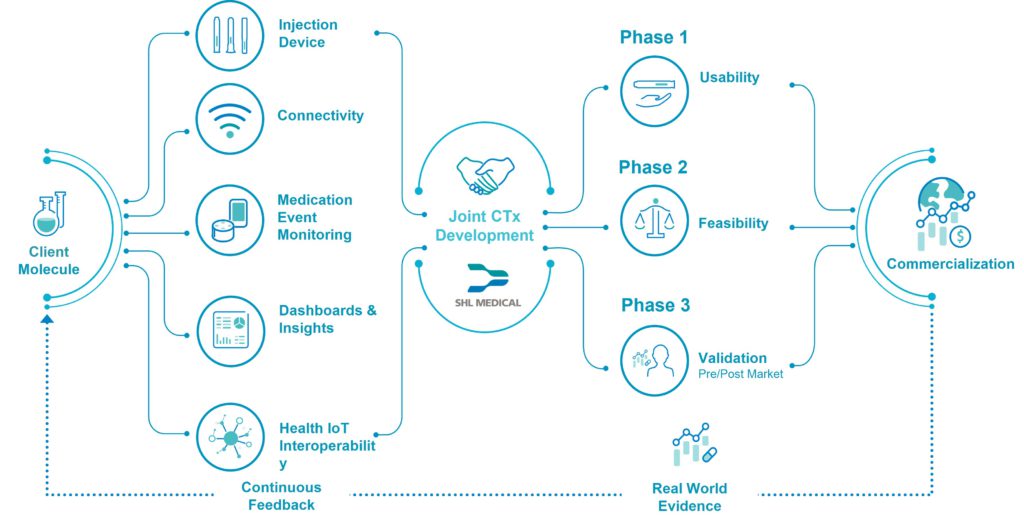
Figure 3: SHL Medical’s conceptual connected therapeutics (CTx) innovation partnership framework.
“It is down to device and pharma partners to grasp the digital healthcare opportunity together and take the next vital steps in building a connected future that promises so much for patients.”
In the drug delivery industry, there are concepts for hybrid mechanical delivery devices, such as diabetes pens or inhalers, which are complemented by digital add-ons. The innovation partnership provides a platform for legacy autoinjectors to mature into the digital space with connected features, interoperable capabilities and a supportive ecosystem to bring the co-developed solution cost-effectively from concept to market.

Figure 4: SHL Medical’s demonstration kit, a proof-of-work in connected healthcare intended to facilitate the development of a digital health ecosystem – from theory into reality.
With digital health technologies evolving at pace, a fundamental aspect of this partnership model is the implementation of the theory within real-world collaborations that have productive outcomes. SHL Medical has identified that an attractive first step in implementing an innovation partnership is through the design and support of a clinical trial, where a connected device can be used to monitor and measure adherence accurately among candidates. By automating the immediate collection and delivery of robust real-time data, connected devices are a valuable enabler on the digital transformation journey while supporting the integrity of the trial in the context of the growing prevalence of decentralised, digitised trial environments. For pharma partners, this approach presents a self-contained vehicle for assessing the power of connected devices together under realistic timeframes, within rigorous conditions and with relatively low risk. This paves the way for further iteration as the partnership evolves.
As part of its work to support pharma partners in progressing their digital healthcare ambitions from concept to commercialisation, SHL Medical has also created connected device demonstration kits that are designed to help inject momentum into early-stage development projects. The contents of the kit encompass the device, interface and even study protocols, and it is made available on a shared-cost basis. They are designed to provide a practical, accessible route to conducting exploratory feasibility studies or mock trials, transposing the theory of digital transformation into real-world implementations that do not involve patients but can form valuable first steps on a journey to develop more sophisticated digital healthcare solutions in the future (Figure 4).
As technical complexity grows and the partnership is nurtured, co-exploration and integration with other health technologies is possible to build more layered platforms rather than independent connected products. An integral part of the DHIE, therefore, is the requirement for strong partnerships between medical technology and device manufacturers and their pharma counterparts, whose digital agendas need to cohere around pharmaceutical innovation. Collaboration should also address interoperability and privacy concerns – and ensure that the aim of digital transformation for each stakeholder group is to improve care and health outcomes for all.
FUTURE DEVICES FOR FUTURE GENERATIONS
For patients, the concept of an injection was transformed by the advent of autoinjectors, allowing self-administration in the comfort of their homes. We are now embarking on a new point of digital transformation for injectables, where drug formulation and device are enmeshed within a connected ecosystem powered by patient data. This opens the door to a range of new possibilities that can now begin to be realised through innovative collaboration.
This is both an exciting prospect for patients, who will be increasingly empowered in the self-management of their own health in their own homes, and an exciting opportunity for pharma companies, which have the potential to instigate such change through a commitment to innovation and collaboration with leading stakeholders across the industry who are aligned on this journey.
The Silicon Valley mindset is unlikely to govern decisions around healthcare. However, the fact that the unexpected catalyst of a pandemic provided a tipping point for significant digital transformation underlines the need for innovation to be twinned with momentum to affect change. It is down to device and pharma partners to grasp the digital healthcare opportunity together and take the next vital steps in building a connected future that promises so much for patients. This is where SHL Medical is leading the way – trailblazing efforts to make the drug delivery ecosystem smarter.
REFERENCES
- Bestsennyy O et al, “Telehealth: A quarter-trillion-dollar post- COVID-19 reality?” McKinsey & Company, Jul 9, 2021.
- Iyawa GE, Herselman M, Botha A, “Digital Health Innovation Ecosystems: From Systematic Literature Review to Conceptual Framework”. Procedia Comput Sci, 2016, Vol 100, pp 244–252.
- Iyawa GE, Herselman M, Botha A, “Identifying Essential Components of a Digital Health Innovation Ecosystem for the Namibian Context: Findings from a Delphi Study”. EJISDC, Dec 5, 2017.
- Rafiei R et al, “Digital Health Integration Assessment and Maturity of the United States Biopharmaceutical Industry: Forces Driving the Next Generation of Connected Autoinjectable Devices”. JMIR Mhealth Uhealth, 2021, Vol 9(3), e25289.
- Goldsack JC et al, “Verification, analytical validation, and clinical validation (V3): the foundation of determining fit-for-purpose for Biometric Monitoring Technologies (BioMeTs)”. NPJ Digit Med, 2020, Vol 3, p 55.

