To Issue 141
Citation: D’hont B, Reynolds J, “Building Digital Solutions to Meet the Unique Therapeutic Needs of Patients”. ONdrugDelivery, Issue 141 (Dec 2022), pp 34–38.
Benjamin D’hont and Joe Reynolds discuss how the key to maturing the digital health market and increasing user retention of digital health apps is integrating agile app development from the beginning of a therapeutic’s development cycle.
“To create digital solutions that solve usability problems, pharma companies need to incorporate patient engagement from the very beginning of product development.”
Across the healthcare and life sciences industries, adoption of patient-facing digital health solutions is notoriously low. The US Office of the National Coordinator for Health IT has reported that only 40% of patients use the portals connected to their providers’ electronic health record (EHR) systems.1 Researchers from the University of California San Francisco have found that only 10% of patients view, download or transmit their EHRs.2 Meanwhile, the UK’s National Institute for Health and Care Research has estimated that 70% of patients abandon assistive technologies, ranging from digital health tools to memory and mobility aids to remote monitoring devices.3
Many factors explain this lack of adoption. Some patients may feel uneasy about relying on technology to help manage their health, while others may feel they are healthy enough that they would not benefit from assistance. However, the most significant obstacle to adoption is poor design. Difficulties with registration and setup, low-quality feedback, poor usability, questionable reliability, unnecessary features and limited impact on behaviour can all cause patients to stop using a product.4
All too often, this happens because the digital solution has been an afterthought in the product development process. Pharma companies remain steadfast in their focus on developing safe and effective therapies that will gain the necessary regulatory approvals, and rightfully so. However, waiting until the later phases of product development to consider the role of a digital solution in supporting a therapeutic will only result in creating a solution that fails to deliver tangible outcomes.
To create digital solutions that solve usability problems, pharma companies need to incorporate patient engagement from the very beginning of product development. Only then will they be able to understand and accommodate the needs, wants and preferences of patients to provide a value-added solution. Aptar Digital Health and Noble, an Aptar Pharma company, have been working extensively with patients to optimise the design and development of digital solutions and maximise adoption by enhancing patient experiences, which ultimately lead to more positive health outcomes.
PATIENT FEEDBACK MATTERS FOR DIGITAL SOLUTIONS
Any pharma product manager understands the importance of obtaining patient feedback at the right moment in the development cycle. If patient feedback during a Phase III trial indicates that the button on an injectable device is not the right size or should be located in a different spot, it is much too late for that issue to be robustly addressed via a design mitigation solution before market launch. Such discussions need to happen long before the clinical trial phase.
Similarly, product managers know the value of obtaining patient feedback first-hand. While real-world evidence and previous clinical studies both demonstrate the market need for a new product and strengthen the regulatory application submission, product teams must recognise that these are secondary sources of information. Direct patient feedback around efficacy, side effects, ease of use and outcomes provides a much clearer picture as to how a therapeutic will perform once it is on the market.
These same strategies for developing a therapy should be applied to developing the digital health solution that accompanies it, with patient involvement right from the very beginning and feedback gathered throughout the development cycle. Adopting this approach has three clear benefits (Figure 1).
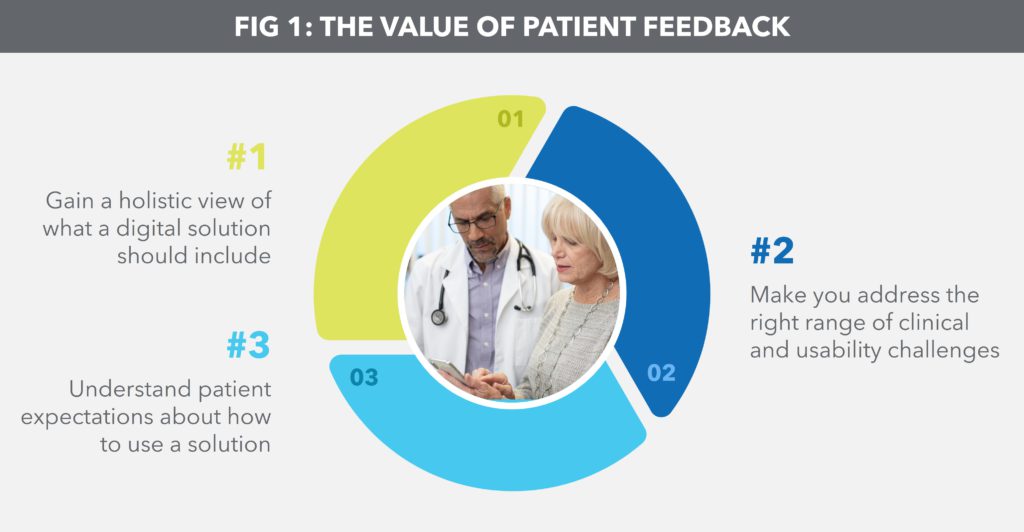
Figure 1: The value of patient feedback.
“The goal of patient involvement throughout the digital health development cycle is to understand patient needs, wants and preferences for using the solution alongside their therapy.”
The first benefit is gaining a holistic view of what a digital solution should include. Adopting a piecemeal approach midway through product development increases the likelihood that a solution will focus on an individual pain point, such as patient anxiety around self-injection or poor long-term adherence. By looking at the big picture, product managers can collect more patient data and gain better insight and perspective into the overall needs of patients.
The second benefit is striking a balance between an n = 1 and an n = all digital solution. The former may be highly customisable, but can be difficult to scale, while the latter may be too general-purpose to meet specific needs. For example, frequent reminders to encourage adherence may alienate patients who are already fully compliant. Gathering feedback throughout product development ensures that the digital solution is designed to address the right range of clinical challenges faced by patients and to address them in the right context.
The third benefit is understanding patient expectations about how to use a solution. It is no secret that e-commerce, social media, travel and banking have set high standards for mobile app design. The more that pharma product development teams talk to patients about what makes them want to use an app, the more likely it is that they will design a digital solution that patients will actually use.
ENGAGE PATIENTS THROUGHOUT DESIGN AND DEVELOPMENT
The goal of patient involvement throughout the digital health development cycle is to understand patient needs, wants and preferences for using the solution alongside their therapy. This is especially important for patients managing chronic conditions or rare diseases, who are often prescribed therapies that they must take for the rest of their lives – and whose clinical outcomes and quality of life are significantly impacted by their ability to self-manage a care plan.
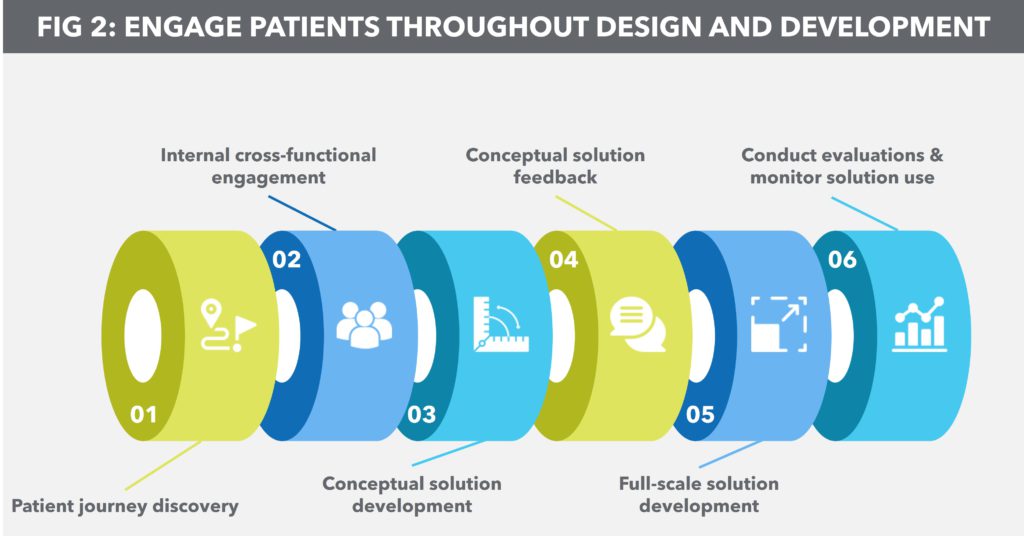
Figure 2: Engage patients throughout design and development.
“This upfront investment in time, energy and resources to support iterative development pays off in the long run as it greatly reduces the likelihood of large-scale changes that require restarting the entire development process further down the line.”
Aptar Digital Health and Noble’s experience shows that knowing what patients are looking for ensures that the digital solution is easy-to-use, offers feedback in a way that positively impacts patient behaviour, provides trusted and reliable medical information and encourages ongoing interaction. Reaching this level of understanding depends on a six-step process (Figure 2):
- Patient journey discovery: The goal of this step is to identify barriers for patients in managing their condition and to understand the clinical and non-clinical factors that drive their decision making. This study should begin as soon as a product team is considering a molecule for development.
- Internal cross-functional engagement: After gathering patient feedback, product teams should convene a multidisciplinary group of key stakeholders to discuss the findings. The goal here is to look holistically at patient needs – therapy-specific, injection anxiety, symptom tracking, adherence, etc. – and discuss the user experience elements and app workflows that would best address these needs.
- Conceptual solution development: Solution development should only begin once internal stakeholders have processed patient feedback and brainstormed general user experience design and workflow. Furthermore, the solution should not be a fully baked product at this stage; the idea is to create a sort of prototype for those inside and outside the organisation to react to.
- Conceptual solution feedback: Obtaining feedback early on will help product managers ensure that not only are the primary needs of the patient being met, but that they are addressed properly within the context of the digital solution. This step also allows for concerns to be addressed or errors to be corrected before full-scale development commences.
- Full-scale solution development: Digital solution development should take place as soon as the clinical development of a therapeutic begins. This helps to ensure that patient needs remain at the centre of the development process. This also aligns the solution with clinical care models for prescribing, administering and tracking the use of a given therapeutic.
- Conduct evaluations and monitor solution use: With regulatory approval as the goal, the digital solution should be subject to the same types of human factors studies and evidence-generation strategy as the therapeutic itself. Likewise, any problems with the solution – from bugs in the software to broken workflows or erroneous information – need to be addressed before the next evaluation phase begins.
ADDRESSING ONBOARDING, ADHERENCE AND MAINTENANCE AT A PERSONAL LEVEL
The digital solution that emerges from the iterative design and development process described above should cover the key moments of taking a therapeutic – onboarding, adherence and maintenance. The solution should provide a personalised experience while also addressing some of the most common challenges that the patient faces when managing a given condition.
Onboarding
Qualified videos and interactive support modules can enhance physical training kits and give patients easy-to-use reference materials. Solutions should account for previous treatments that a patient may have used and consider important differences, such as storage, injection angle or frequency of dosage. By reducing errors associated with patients applying their previous experience to a new therapy, digital solutions can help avoid adverse outcomes.
Adherence
Here, the balance between n = 1 and n = all is critical. A solution should monitor patient behaviour and modify engagements accordingly. For example, notifications should reduce in frequency if adherence is improving to avoid annoying a patient to the point that they stop using a solution altogether. Similarly, if self-injection anxiety is persistent, the solution should alter the tone of notifications or provide more supportive resources.
Maintenance
Enabling patients to monitor their symptoms and report their side effects helps bridge the gap between doctor appointments that may be many months apart, as it provides a continuous record of how a patient has been feeling. An effective digital solution will use this information to help both the patient and their healthcare provider discuss how a therapy is working and whether a care plan should change.
AGILE DEVELOPMENT AND ACCELERATED DE-RISKING
As pharma companies engage patients throughout the digital solution development process, it is possible that the highly agile nature of this approach will clash with the industry’s traditional aversion to risk. Fortunately, this agile approach is purposefully designed to be a counterbalance to risk aversion.
The reason for this clash is because continuously monitoring and building a digital solution throughout therapeutic product development is an example of accelerated de-risking. This upfront investment in time, energy and resources to support iterative development pays off in the long run as it greatly reduces the likelihood of large-scale changes that require restarting the entire development process further down the line. Just as it is too late inPhase III to move the button on a self-injection device, it is also too late in Phase III to revamp the symptom-tracking workflow of a digital solution.
Accelerated de-risking has the additional benefit of aligning with compliance requirements. Again, while the agile process may seem to contradict the rigors of regulatory approval, incorporating continuous improvements to a digital solution, and beginning this process at the product ideation phase, makes it easier to build compliance into the product development lifecycle. Issues can be addressed as they arise, within the parameters of a software “sprint”, long before they become larger problems that pose a threat to regulatory approval.
In more traditional environments, the digital solution is built as a standalone product. Even if the development process follows industry best practices for discovery, journey mapping, userexperience testing and human factors, the finished product is unlikely to align with the specific needs of a particular therapeutic and its patient population. The digital solution may address a pharma company’s business problem – the lack of a patientfacing mobile app – but it is not positioned to solve the patient usability problems that are at the core of poor adherence and worsening outcomes.
Agile development, on the other hand, further ensures that the digital solution and the therapeutic are co-created. Product managers on both teams work towards the same milestones, are part of the same feedback loop and address the same issues at the same time. As a result, the finished product addresses both the business problem and the usability problem, leading to the launch of a digital solution that patients are more likely to use.
CONCLUSION
Patient-facing digital solutions are increasingly popular in the life sciences industry, but the overall market for such solutions remains immature. Most pharma companies are unable to properly execute their vision for a digital solution. All too often, the solution is developed in a silo and regarded as an add-on to a therapeutic already in development. As a result, adoption rates are low and abandonment rates are high.
Equally, the most effective digital solutions are tightly coupled with their therapeutics from the ideation stage of product development. Aptar Digital Health and Noble are respected and experienced industry partners, working with pharma companies to ensure that their digital solutions support each step of the patient journey with a therapeutic, meeting their individual needs and improving their outcomes while creating long-term value for the company.
For more information about Aptar Pharma and Digital solutions, visit: www.aptar.com/resources/building-digital-solutions-to-meet-the-unique-therapeutic-needs-of-patients
REFERENCES
- Johnson C, Richwine C, Patel V, “Individuals’ Access and Use of Patient Portals and Smartphone Health Apps, 2020”. ONC Data Brief, Sep 2021.
- Lin SC et al, “Are Patients Electronically Accessing Their Medical Records? Evidence From National Hospital Data”. Health Aff (Millwood), 2019, Vol 38(11), pp 1850–1857.
- Howard J et al, “Exploring the Barriers to Using Assistive Technology for Individuals with Chronic Conditions: A Meta-Synthesis Review”. Disabil Rehabil Assist Technol, 2022, Vol 17(4), pp 390–408.
- Park JI et al, “Lack of Acceptance of Digital Healthcare in the Medical Market: Addressing Old Problems Raised by Various Clinical Professionals and Developing Possible Solutions”. J Korean Med Sci, 2021, Vol 36(37), Article 253.

