To Issue 146
Citation: Storz J, “Interview – Why Sustainable Packaging Matters in Pharma’s Circular Approach – Aptar Pharma’s Futurity Platform Leads the Way”. ONdrugDelivery, Issue 146 (Apr/May 2023), pp 20–24.
Q Where do you see the biggest opportunities for the pharmaceutical industry to advance sustainability objectives?
A Good question! Industry estimates seem to agree that about 4%–5% of global greenhouse gases (GHGs) come from the healthcare sector,1 with up to one-third of those emissions related to pharmaceutical products.2 To understand where those emissions are coming from, one can look at data provided by the European Federation of Pharmaceutical Industries and Associations. Their members have classified and broken down GHG emissions, where approximately 20% are classified as Scope 1 and Scope 2 and the other 80% are classified as Scope 3 emissions. That means that 80% of the pharmaceutical industry’s GHG emissions are generated in their upstream and downstream value chains.3
“Having common goals with the customer allows us to work co operatively with them to achieve the specified, targeted sustainability goal, while making sure safety and regulatory requirements are covered.”
Pharma value chains are quite complex and therefore Scope 3 emissions must be approached at many different levels to achieve a sustainable reduction of the emissions. However, the industry is highly motivated and actively developing new innovative ways to lower its emissions. This has recently been underlined by a number of major pharma companies that have made a clear commitment to deliver net zero health systems collectively, applied across their supply chains, patient care networks and clinical trials.4 Another initiative gaining traction is the development of the iGAL calculator, which illustrates how green chemistry and engineering innovation can reduce waste during API manufacturing.
From our perspective, a very important approach to lowering Scope 3 emissions is through primary packaging and devices. Primary packaging really matters to patients and consumers as it is the most visible “problem”. They hold the packaging or device in their hands every time they use their medication, and when it’s fully consumed, it is the same patients or consumers that ultimately control how they dispose of it. We strive to make that easy for them by making more recyclable packaging systems.
As primary packaging systems are highly regulated and complex, it will be essential for the industry to pursue common objectives collaboratively to advance sustainability objectives fast enough.
Q What challenges does the pharmaceutical packaging industry face in reducing its CO2 footprint and achieving more sustainable solutions?
A One of the things that is significantly different for the pharmaceutical packaging industry is, again, the level of regulation. It’s a very high bar. The regulations impose some limits on what we can do, especially for primary packaging, as this has direct contact with the drug formulation. Changes to enhance the sustainability of the packaging systems cannot result in reduced product safety or efficacy. Right now, many of the packaging and waste regulations are still being defined and implemented, so there is still some uncertainty around the details. For example, the deadline for implementing new EU regulations for recyclable packaging in the pharmaceutical industry is still over 10 years away. The pharma industry needs this time to create and innovate new compliant packaging solutions that will lead to a more circular approach. New product packaging regulations for recyclability for consumer products will become effective in only two years, by 2025, so they have very little time to solve these challenges. These tight timelines and high expectations for the consumer business mean we have to move in a more sustainable direction immediately. We are focused on innovations in primary packaging and drug dispensing that support the circular approach while simultaneously meeting drug-safety requirements. We believe this dual approach will help us to meet the tight timelines and create value for both the consumer and the planet.
Q Can you explain what some of the main limitations or challenges are with pharmaceutical primary packaging meeting sustainability objectives?
A Drug delivery systems are often complicated and made of many individual components, composed of different materials, all designed to work perfectly together to deliver drug products to patients with precision and accuracy. These are systems like nasal sprays, inhalation devices and semi-solid dispensers. The pharmaceutical regulations for these types of primary packaging systems are both strict and comprehensive, posing challenges to quickly adapting them to meet sustainability targets. In the past, material composition was defined by safety and use requirements, which were clearly documented for regulatory filings. Changing or replacing materials with more sustainable materials isn’t just a simple switch. The implications are more significant for pharmaceutical packaging. Changes may require new assessments if safety requirements are fulfilled, such as extractables or leachables testing, and associated regulatory documentation may need to be updated. Our experience and capabilities allow us at Aptar Pharma to support customers with required testing and documentation. Having common goals with the customer allows us to work co-operatively with them to achieve the specified, targeted sustainability goal, while making sure safety and regulatory requirements are covered. This support will be critical for the industry to be able to deliver sustainable next-generation drug delivery systems on time.
Q How do consumers feel about the importance of recycling or sustainability with respect to pharmaceutical packaging?
A Consumers hold the packaging in their hands every time they use the product. They see and touch the packaging materials and will ultimately decide if the packaging system will be recycled or not (Figure 1). As they are directly involved in the decision process, their views on the importance of sustainable packaging systems is critical for us. Our surveys clearly show us that consumers have a strong preference for products that come in packaging that can be recycled.

Figure 1: In the end, the patient or consumer decides on how or if a device is recycled.
“Aptar entered a strategic partnership with PureCycle in 2019 to bring forward the use of ultra-pure recycled polypropylenes into Aptar’s dispensing solutions for food-grade applications.”
Consumers are increasingly paying more attention to how they produce waste and recycle packaging. Most consumers are willing to take an active role in recycling, but they still need some additional education to make it more effective. For example, most consumers perceive that a device made of both glass and plastic parts is more recyclable than a full-plastic monomaterial unit. But this is incorrect. Even in locations with high consumer awareness of longstanding recycling practices, current recycling processes can fail when the consumer is unaware of the best way to dispose of or recycle the complex packaging components of the emptied unit.
Therefore, we’ve really reinforced the importance of making our products easy to recycle. With our sustainable product solutions, consumers can put the entire emptied device in an existing recycling stream without any disassembly or separation effort. This guarantees that most components find their way to the right recycling path and stay in the circular process as long as possible. We also find that having a device that is clearly labelled or certified regarding its recyclability makes a measurable difference in the frequency of it being recycled effectively by the consumer.
Q What is circularity and how is Aptar committed to applying the circular economy model?
A Circularity considers the overall expected lifecycle of a product and incorporates sustainability optimisations at the design stage. In a circular economy, when the consumer is finished with a product, it is returned to the supply chain to be recycled, reused or repurposed and not put into landfill (Figure 2). As material resources are finite in nature, we must consider a product’s entire lifecycle to enhance its circularity in practice. We all need to understand that we will need to use what was once considered “waste” as a valuable resource moving forward in order to achieve circularity.
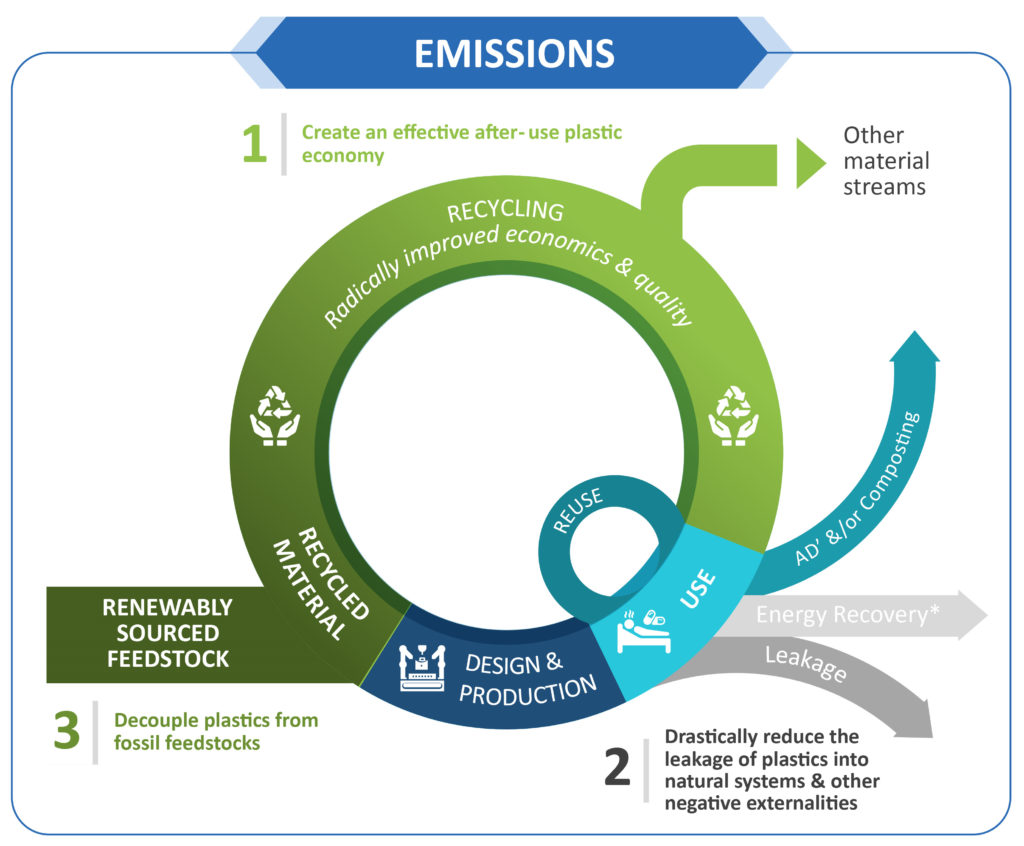
Figure 2: Aptar Pharma is aligned with the Ellen MacArthur model for circular economy in the plastic packaging industry, which includes reduction of plastic
leakage, as well as minimising the use of fossil feedstock. Based on this model, reduction of emissions through recycling, reuse, replacement of materials and
sustainable product design is evaluated.
Beyond efficient handling of our resources, circularity is important to keep the environment cleaner. Successful circularity will see improved quality of materials, including recyclates, and reuse of materials. To date, using post-consumer resins (PCRs) gained by mechanical recycling is still rarely an option for primary packaging components in pharma, at least when the components are in direct contact with drug formulation. However, using innovative processes, such as chemical recycling to generate clean PCR recycled materials, needs to be further developed and scaled up.
Another approach to generating high quality recyclates from recycling streams is PureCycle Technologies’ (FL, US) patented recycling process. Aptar entered a strategic partnership with PureCycle in 2019 to bring forward the use of ultra-pure recycled polypropylenes into Aptar’s dispensing solutions for food-grade applications. Such processes could also prepare the future for the use of recycled materials in primary packaging for pharma, and we need to look together with regulatory authorities into furthering such solutions.
We need to be better at reusing and recycling materials, as well as improving the way we are designing products and packaging systems right from the beginning. It will be crucial to develop systems using fewer materials, eliminating recycling disruptors and minimising the number of different materials used in a device.
Q How does Aptar Pharma effectively design sustainability into its products?
A Aptar Pharma takes an integrated approach to incorporating sustainability into every new device or technology system. We have already implemented the use of our proprietary EcoDesign tool, which incorporates lifecycle assessment (LCA) functionalities into the process. This allows us to assess how our packaging systems will impact the environment before we ever make them, and we can also use it to assess our existing products. Aptar has committed itself to using EcoDesign tool assessments on every new packaging system we develop moving forward, and to design sustainability into the product development process from the very beginning. We did this in the absence of a standardised EcoDesign tool that was suitable for our industry, so Aptar invested in developing its own EcoDesigntool in co-operation with established LCA authorities that incorporated available standards, considered best practices and ISO standards. Our tool provides relevant, measurable results and enables us to design products that add to a circular approach.
With numerous EcoDesign tools out there, it is difficult for the industry to arrive at a consistent and comparable approach to EcoDesign and LCAs. This inherently poses a challenge for the industry as there is no common way to validate these assessments. We believe that the way forward will be to harmonise the best practices from these various assessment tools into a common approach.
“Because sustainability has become an important company-wide objective at Aptar Pharma, we decided to use the term Futurity to represent our sustainable solutions platform.”
Q What is Futurity™ and why is it important to Aptar?
A Because sustainability has become an important company-wide objective at Aptar Pharma, we decided to use the term Futurity to represent our sustainable solutions platform (Figure 3). This new branding will help our customers and consumers easily identify products and technologies from Aptar Pharma that are designed with enhanced sustainability and circularity in mind. This can include devices designed with enhanced recyclability features, those using lower global warming potential (GWP) propellants, mono-material construction or other impactful design features.
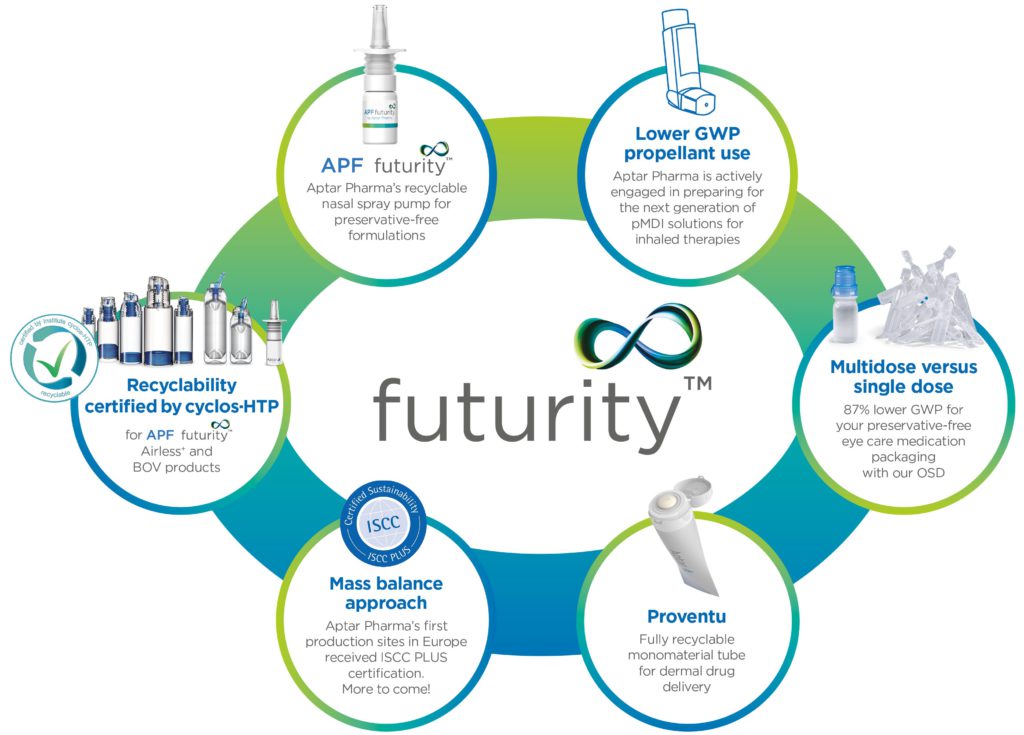
Figure 3: Aptar Pharma’s growing platform for more sustainable solutions, Futurity™, comprises drug-delivery solutions and services that enable reduction of emissions through increased recyclability, use of circular materials, reduction of packaging materials, reduced waste or other means.
These are not simple solutions. For example, changing from a traditional HFA propellant in a pressurised metered dose inhaler (pMDI) to a newer one with lower GWP is a complex affair. This can involve modifications to the pMDI container closure system, including the metering valve itself, but beyond that, the new propellant must be assessed for safety and toxicology, and the final formulation may need to be optimised or redeveloped to ensure appropriate formulation stability.
The propellants currently used in pMDI devices for asthma or chronic obstructive pulmonary disease applications may be a significant contributor to the CO2 footprint of these products for pharmaceutical companies. Therefore, changing them can result in major reductions in CO2 output and is worth the effort. We work very closely with our customers to support this critical conversion process, both in the lab and with our regulatory teams, to make the switch easier and faster for them.
We also work on drug delivery solutions that incorporate circularity, such as replacing conventional materials with circular resins, waste reduction, packaging reduction and improved recyclability, to which we apply LCAs and external validation of recyclability claims.
Q What is APF Futurity™ and what makes it a more sustainable packaging technology?
A The Advanced Preservative Free (APF) Futurity is our latest multidose nasal spray system (Figure 4). With the support of our EcoDesign tool, we were able to create a highly recyclable nasal spray system that maintains all the reliable functionality and precise dosing of our traditional APF system. APF Futurity is metal-free, using a polyolefin-only material mix. Nasal sprays usually contain metal springs or even stainless steel balls in their pump and dosing mechanisms. Without metal components, a straightforward recycling of the pump is possible, ensuring higher quality recyclates.
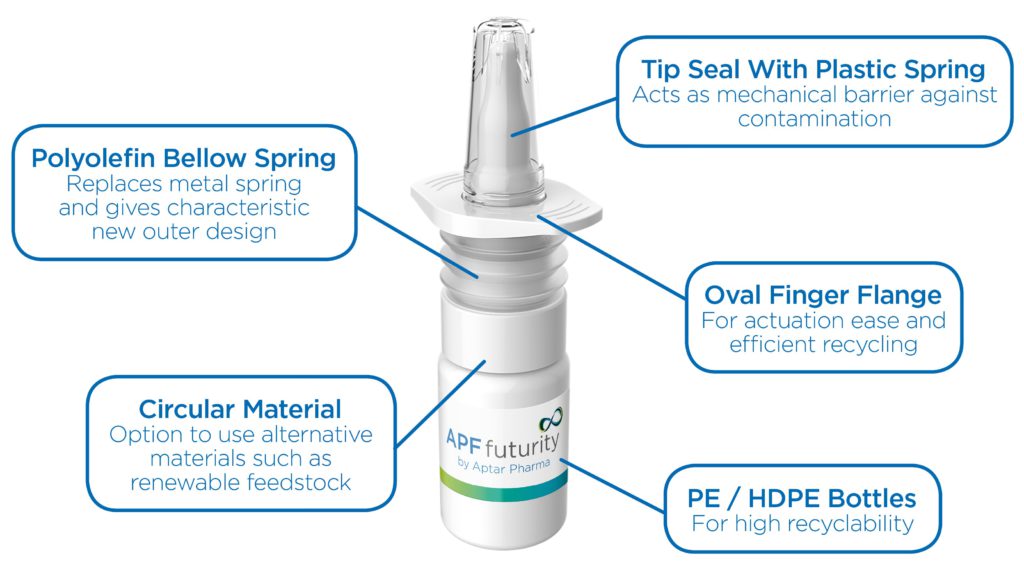
Figure 4: APF Futurity, metal-free nasal spray pump is designed for easy recycling by the user, and is certified highly recyclable by cyclos-HTP.
One of my favourite features is the new oval finger flange because it increases the likelihood that the product gets into the right recycling stream. Round components often roll away after the infrared (IR) scanner detects them on the recycling belt. This can lead to items being sorted incorrectly, which has an impact on the quality of recyclates. The new oval flange feature stops the device from rolling and therefore results in it being correctly sorted as the IR sensors trigger air streams that are able to hit the nasal spray more accurately.
In a nutshell, the APF Futurity was designed for easy recycling. Consumers do not need to disassemble or separate it into parts – they can place the emptied device as is into the regular local recycling stream.
Q Can you provide an outlook on Aptar Pharma´s way of moving forward in terms of sustainability?
A Our focus on sustainability is always driven by customer needs and our own objectives. Whether they are looking for a reduced CO2 footprint, increased recyclability or reusable/refillable solutions, our sustainability efforts tend to align with their common objectives. Obviously, our customers help to direct our sustainability objectives. We also pay close attention to what consumers are telling us they want with respect to primary product packaging and sustainability, and we ask them directly through surveys and primary research. Every day consumers are becoming more aware of the importance of sustainability in their lives and are demanding that the products they buy reflect that. All of this is done under the overarching considerations of the regulatory bodies to ensure continued compliance with changing requirements.
So how will Aptar Pharma achieve this in practice? We will continue to use our EcoDesign and LCA tools to design enhanced sustainability into all our new packaging systems and optimise our existing products wherever possible. This allows us to consider the critical sustainability aspects of the design from the earliest stages of development, ensuring that we maximise positive impact throughout the entire product lifecycle. I’d like to add that we are using state-of-the-art materials and methods that will move us closer to achieving our larger sustainability goals and ultimately move our products fully into the circular economy. Stay tuned, there is more to come.
For more information about APF Futurity™, visit: www.aptar.com/products/pharmaceutical/apf-futurity-nasal-spray-recyclable
For more information about Aptar Group’s commitment to sustainability, visit: www.aptar.com/esg
REFERENCES
- “Health Care’s climate footprint”. Health Care Without Harm. Climatesmart health care series. Green Paper Number One. Sep2019.
- “It’s Time to Calculate the Carbon Footprint of Pharmaceutical Products”. The Medicine Maker. Nov 30, 2022.
- “How research pharmaceutical companies are contributing towards tackling global warming”. EPFIA, Nov 22, 2022.
- “Seven pharma CEOs announce new joint action to accelerate net zero healthcare”. Press Release, AstraZeneca, Nov 3, 2022.

