To Issue 146
Citation: Wooller T, “Sustainability in Drug Delivery: How do we get there?”. ONdrugDelivery, Issue 146 (Apr/May 2023), pp 14–17.
Tim Wooller discusses the urgent need to transition away from disposable models of drug delivery device design and move towards reusables, providing insights into the reasons why and the methods by which this can be achieved.
Sustainability has become an increasingly hot topic for pharmaceutical companies and medical device manufacturers in recent years, with many setting themselves ambitious goals to dramatically reduce their carbon footprints. Pfizer, for example, has pledged to become carbon neutral and reduce the company and its value chain’s emissions by 90% by 2040,1 while Johnson & Johnson aims to obtain 100% of its electricity from renewable sources by 2025.2
There are multiple reasons why companies in this sector are making such pledges. First, there is the acknowledgement of the huge impact the healthcare sector has on the environment – believed to be 4.4% of worldwide greenhouse gas (GHG) net emissions and five million tonnes of waste per year.3 Healthcare companies have been galvanised to respond to this challenge because of the direct link between poor health and climate change. Put simply, to better serve their patients, healthcare companies have a responsibility to address their own environmental footprints.
“While disposable devices are of great benefit to patients, pharmaceutical companies and healthcare systems, there are very good reasons why steps need to be taken right now to phase out disposables in favour of reusables for many therapies.”
Secondly, it can be directly observed in user studies that patient opinion is rapidly changing to include a demand that this topic be addressed. For those who self-administer their therapies at home, the constant filling and disposal of sharps bins acts as a tangible reminder of the issue at hand.
Finally, the scientific consensus and resultant government regulation are only headed in one direction, so it is important for many businesses within healthcare to be proactive rather than reactive to the situation. For an industry renowned for its cautious pace and pragmatism, the future is fast approaching, and the sense of urgency is increasing with it. Most recently, a UN climate change report urged that “inaction and delays are not listed as options”.4
While it would be naïve to say that achieving sustainability in the healthcare sector will be easy, particularly given the industry’s reliance on disposable materials, it is in no way an impossible feat. Instead of tackling the healthcare industry as a whole, which can be daunting, looking at each area individually makes it appear a much more achievable and realistic goal. It’s also encouraging to see that most doctors and nurses want to help hospitals reach net zero, with only 12% of respondents saying they did not have the time or resources to be involved.5
Looking to drug delivery in particular, the use of disposable autoinjectors and pens has increased rapidly in recent years. While disposable devices are of great benefit to patients, pharmaceutical companies and healthcare systems, there are very good reasons why steps need to be taken right now to phase out disposables in favour of reusables for many therapies.
In order to fully understand where to go and what to do to get there, it’s also important to understand the progress made to date. This article will explore all of these elements with a focus on injectable drug delivery, providing tangible guidance for pharmaceutical companies and manufacturers on the best way forward.
“Before embarking on any significant sustainability initiative, it is critical to take a step back and understand the broader system, starting with the patient and therapy journey.”
WHAT PROGRESS HAS BEEN MADE TO DATE?
Autoinjectors and pens that support the subcutaneous self-administration of a range of biologic drug therapies have become a popular choice for patients who struggle to use prefilled syringes. They are most commonly used at home to treat chronic conditions, which often impact mobility, giving patients greater independence and confidence to adhere to their treatments. Naturally, greater adherence benefits pharmaceutical companies, but autoinjectors and pens also provide an important means of differentiation for off-patent drugs to defend against biosimilars, or for biosimilars to compete with established brands when coupled with a compelling device design.
Healthcare systems also benefit by reducing the number of hospital visits patients need to make.
Because of this, the use of autoinjectors and pens has increased exponentially, with the most popular type being prefilled, single-use disposables. This has happened for a variety of reasons; for example, the initial financial outlay for each unit is relatively low compared with some of the more complex reusable devices on the market. They are also simple and convenient to use and, because they require a minimal number of user steps, they can often be perceived as lower risk.
In terms of sustainability progress made within the industry, there have been some interesting developments. The “Alliance to Zero” is a collective of eight founding companies that aims to transition the pharmaceutical supply chain to meet the net zero targets set out by the Paris agreement. Ypsomed’s Ypsomate Zero disposable autoinjector claims to be the first carbon-emission-free prefilled autoinjector,6 which it achieves through a combination of using alternative polymers, value-chain optimisation and carbon offsetting. These developments have provided a crucial foundation for the industry to build upon. However, it’s evident that progress in this area needs to be accelerated to effect greater tangible change.
CONSIDERING THE USER AND THERAPY JOURNEY
Before embarking on any significant sustainability initiative, it is critical to take a step back and understand the broader system, starting with the patient and therapy journey. Using a range of human-centred-design (HCD) methods, such as stakeholder and user journey mapping, contextual enquiry and creative workshop techniques, it is possible to frame the problem in the correct way by asking some fundamental questions, such as:
- How will the drug-device combination be used?
- What steps are required throughout the process of prescription, supply, preparation and use?
- Will the drug require regular or infrequent injections?
- What effect does the patient’s condition have on their ability to use a device?
The answers to these questions will define the right approach and application, saving unnecessary time and material in the process. For example, therapies that only require infrequent injections may be best administered by a healthcare professional without the aid of any device beyond a syringe.
HCD methods can also be used all the way through the development of a device to ensure that intended uses are optimised and adhered to and that unintended uses, which may have an impact on sustainability, are avoided.
RETHINKING THE BUSINESS MODEL – THE PATH TO REUSABLES
Consider a drug therapy that requires weekly injections, spread across just two years – this would require 100 disposable devices or a single reusable autoinjector (Figure 1). Excluding the disposable elements common to both these types of injectors, such as glass drug cartridges, needles and secondary packaging, there is a considerable amount of material, such as plastic enclosures and mechanisms, that find their way to incinerators or landfills with each injection using the disposable model. Removing this material in a value chain right from the outset has significant positive environmental impact. Similar to other industries seeking to remove single-use plastics, healthcare systems must take these important steps.
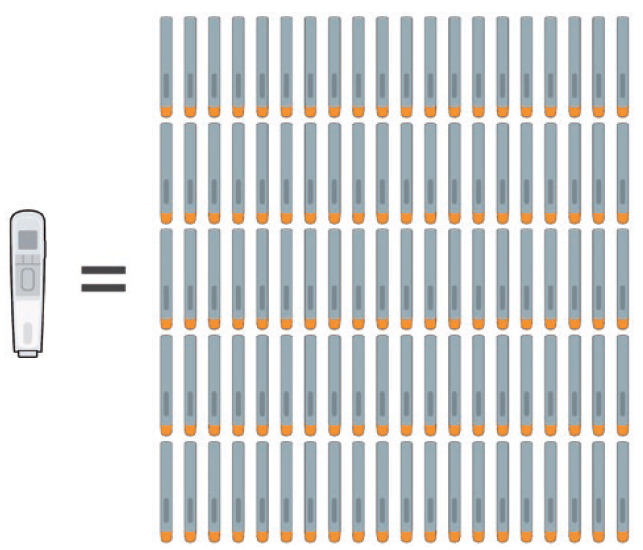
Figure 1: A therapy requiring weekly injections over the course of two years would need either 100 disposable single-use autoinjectors or a single reusable one.
“Once the decision has been made to move towards reusable devices, there is great potential to add many patient-centred features and functions that simply aren’t practical if the device is disposed of every time.”
Once the decision has been made to move towards reusable devices, there is great potential to add many patient-centred features and functions that simply aren’t practical if the device is disposed of every time, including:
Comfort features: reusables don’t need to be quite as slimline and basic as disposables. Their forms can have more overt and optimised features to aid usability, which would be deemed wasteful in disposables.
Automation: one way to mitigate the fact that disposables are pre-primed is for reusables to use motor systems. As well as expelling the drug, motor systems can aid comfort by offering adjustable settings, such as injection depth, injection speed and dwell time, to overcome some of the issues patients face when injecting. A great example of a product already in the market with these features is easypod® by Merck.
Electronics: reusable devices, especially if motor driven and already containing batteries, can include sophisticated electronics encompassing sensors, graphical user interfaces and connectivity, opening up a world of additional benefits to the patient.
Combining these features can result in a better product with better patient engagement which can aid adherence. This in turn can encourage brand loyalty of great benefit to pharmaceutical companies in the longer term.
CONSIDERING THE ENTIRE VALUE CHAIN AND PRODUCT LIFECYCLE
In addition to the efficiencies and benefits achieved by reusable devices, it is important to consider the value chain and product lifecycle (Figure 2). R&D can employ methods to make an efficient product, but is the greater business run with environmental considerations in mind? Clinical trials generate a lot of medical waste – how can this be reduced? Has the manufacture of both the drug and device been optimised to be as efficient as possible? What about distribution and storage – what efficiencies can be achieved here? Finally, do suppliers to the pharmaceutical company or medical device manufacturer share the same environmental goals? There is a range of methods and guiding principles which can help answer these questions, including lifecycle assessments (LCAs), design for optimisation, design for efficient distribution and design for optimised end of life and disassembly.
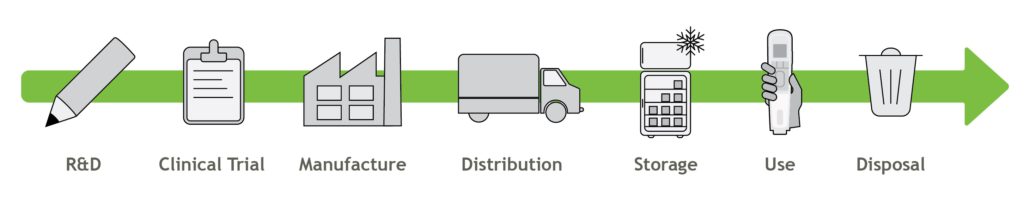
Figure 2: The value chain of an autoinjector.
“This is not the time to tinker at the edges and seek marginal gains – the reliance on single-use devices has to be significantly reduced.”
Lifecycle Assessments
LCAs enable companies to assess their entire value chain by entering data and getting back actionable results. Sometimes the results are surprising once all aspects of the lifecycle are considered. One very good example is when drug therapies require refrigeration – this has major impacts all the way through the value chain, from manufacture, storage and distribution to end use. Storing a disposable injector and its packaging in a patient’s refrigerator has a far greater impact than just a drug vial; it takes up more space and a greater proportion of the refrigerator’s energy budget. It is important, therefore, for cold-chain logistics to consider everyday use as well as distribution and storage.
LCAs don’t just find efficiencies for existing products and systems; they are also an essential tool to help develop new products. They do, however, have their drawbacks. The richer the data that is inputted, the better the result. The opposite is equally true – there can be major shortcomings in comparing the value chain of design concepts with existing products if a range of inputs is unknown at the conceptual stage. It is difficult to establish how concepts might be manufactured, distributed and stored until these factors are far more resolved downstream. A better approach is to “compare apples with apples” and use LCAs as a tool when considering multiple early concepts against each other using a range of educated assumptions.
Design for Optimisation
Recent progress in artificial-intelligence-driven generative tools has allowed developers to design, analyse and simulate designs that require less material and energy to manufacture with increasing speed, accuracy and confidence. Selecting materials with a lower environmental impact is another consideration. There are now many US FDA-approved biodegradable polymers to choose from for medical applications, so the options open to developers to optimise their products are greatly increased.
Design for Efficient Distribution
Once manufactured, how might the product be most efficiently transported? The most impactful upstream consideration is to locate manufacture as close as possible to where the product is marketed. The next best solution, if the first isn’t commercially viable, is to consider package, carton and crate proportions, and to design devices and secondary packaging to nest in the most efficient way possible to eliminate empty air during transport.
Design for Optimised End of Life and Disassembly
Designing the end of life for a product is increasingly important to ensure circularity or “cradle-to-cradle” compatibility. First, limiting the number of different materials used in a product greatly aids sorting and recycling efforts at its end of life. Second, providing a clear path to disassembly is critical. With various levels of “right to repair” legislation now implemented in the US, EU and UK for consumer goods, it makes sense for healthcare to follow that lead. Given that medical devices are generally not user-serviceable, this would have to take the form of an end-of-life return process set up by the manufacturer.
THE WAY FORWARD
As the urgency regarding the climate and the environment gathers pace, the tools and methods discussed, such as considering the user and therapy journey, value chain and lifecycle, provide a way forward for pharmaceutical companies to take more effective steps to reduce waste and emissions.
This is not the time to tinker at the edges and seek marginal gains – the reliance on single-use devices has to be significantly reduced. When it comes to single-use materials, the issue of hazardous waste will hamper sustainability efforts in hospitals, there is less of a barrier for patients using autoinjectors for at-home self-injection. Pharmaceutical companies and medical device manufacturers have much to gain by balancing patient, planet and purpose, and by aspiring to develop more durable and engaging products that promote adherence in the process.
The author would like to thank Luke Robbins, Senior Consultant – Industrial Design, for his contribution to this article. Mr Robbins leads sustainability initiatives at PDD.
REFERENCES
- “Environmental Sustainability”. Pfizer webpage, accessed Mar 2023.
- “Our Environmental Sustainability Approach”. Johnson & Johnson webpage, accessed Mar 2023.
- Budd K, “Hospitals race to save patients — and the planet”. AAMC, Oct 2019.
- “Flagship UN report extolls win-win water partnerships to avert global crisis”. UN News, Mar 2023.
- “Do no harm: Healthcare professionals address sustainability and climate change”. Economist, Sep 2022.
- “Zero carbon emission pre-filled autoinjector”. Company Web Page, Ypsomed, accessed Mar 2023.
Previous article
IMPROVING RECYCLABILITY IN DRUG DELIVERYNext article
DESIGNING SUSTAINABILITY INTO PHARMACEUTICAL DEVICES
