To Issue 171
Citation: Biasio L, Skibba G, “Sustainable GLP-1 Drug Delivery: Future-Proofing Therapy Administration for Obesity and Beyond”. ONdrugDelivery, Issue 171 (Apr/May 2025), pp 14–19.
Lorenzo Biasio and Gloria Skibba discuss the integration of Ypsomed’s Net Zero Program into its self-injection platforms and explain how YpsoMate 1 and 2.25 mL autoinjectors have been redesigned to achieve a lower carbon footprint. With the evolution of GLP-1 therapies and the exploration of higher-dose formulations, the demand for higher-volume autoinjectors is increasing; this article explains how Ypsomed has also applied its sustainability principles to larger-volume delivery.
Almost 20 years ago to the day, the glucagon-like peptide-1 (GLP-1) era began. On the heels of basic research on the venom of Gila monsters in the preceding decades,1 exenatide secured the first US FDA approval in the class on April 28, 2005.2 While the prominent GLP-1 brand names are household names today, the class was off to a relatively slow start at first – five years passed before it reached blockbuster status (US$1 billion (£770 million) in sales), and another five went by before the first formal approval of a GLP-1 drug indicated for obesity became a reality.3
Last year saw sales for the class cross $50 billion and, with another 40% in revenue growth in store for 2025, the GLP-1 segment of the pharmaceutical industry is firmly in the rapid growth phase of the market, per Investment Bank Consensus. On the same measure, cumulative revenues across the major pharmaceutical companies alone are projected to reach close to $165 billion by 2030 (Figure 1).
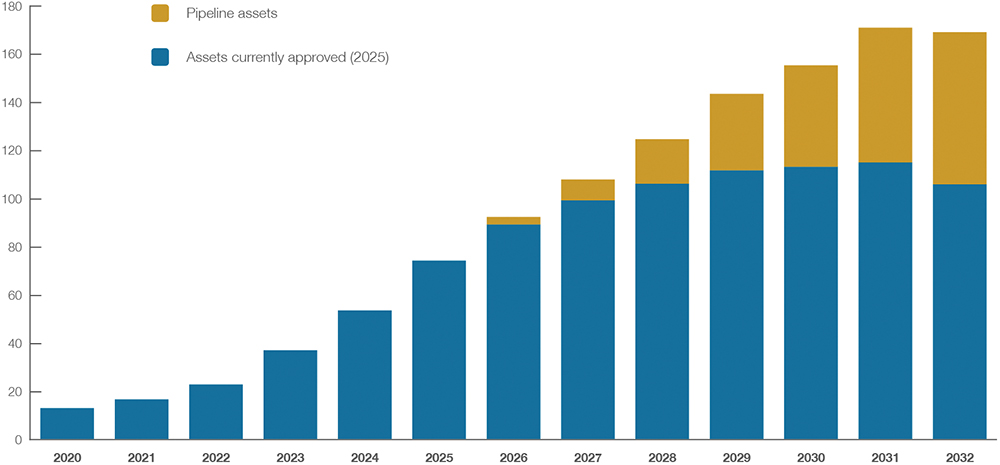
Figure 1: Projected growth of the GLP-1 market (2020–2032): Capital Market Consensus estimates in US$ billion, highlighting the contribution of currently
approved assets (as of 2025) and pipeline assets expected to enter the market. Source: Investment Bank Consensus via DrugAnalyst, Company reports.
Note: Does not include biosimilars.
While GLP-1s are mainly known for their impact on glycaemic control and weight management, they play a role beyond, with effects confirmed – or at least suspected – on cardiovascular risk, kidney disease, obstructive sleep apnoea and osteoarthritis. Even otherwise dispassionate media outlets have labelled these drugs “miracle drugs” that will “change the world”. Considering the diversity of clinical trials and indications they are tested in – including the notoriously challenging Alzheimer’s disease – these claims may well hold a substantial amount of truth (Table 1). After all, there are over 100 originator drugs and almost as many biosimilars in the pipeline, many of which will never progress to commercialisation, but some of which will further push the boundaries on patient convenience, efficacy and indication spectrum.
| Currently approved conditions | Conditions under clinical investigation/in registration |
| Cardiovascular risk reduction Chronic kidney disease Obesity Obstructive sleep apnoea Type 2 diabetes |
Alzheimer’s disease Atherosclerosis Diabetic retinopathy Heart failure Ischaemic stroke MASH Type 1 diabetes |
Table 1: Snapshot of GLP-1 therapeutic indications – currently approved conditions and those under clinical investigation or registration. Source: Pharmacircle/Prescribing information; subject to change.
Today, Novo Nordisk estimates that just under 20 million people are treated with GLP-1 drugs.4 Given a well-stocked pipeline, a substantial global addressable population (the obese population alone is projected to exceed 1.2 billion by 20305), and in light of the very beneficial clinical profile of these drugs, it is plausible that over 100 million patients worldwide will be treated with GLP-1s in the near future. Particularly with the expected adoption boost as biosimilars enter the market towards the end of the decade, affordability barriers may begin to crumble, especially in lower-income regions where GLP-1 access currently remains limited.
EXTENDED DOSING INTERVALS AS A DRIVER FOR IMPROVED SUSTAINABILITY
Native GLP-1 has a half-life that is measured in mere minutes6 and, as such, has limited therapeutic relevance without continuous dosing. Exenatide stretched that measure to about 2.5 hours3 – sufficient for therapy but requiring twice-daily injections that placed a significant burden on patients. Even with the improved convenience of once-daily options, the true breakthrough in adherence came with current-generation, once-weekly GLP-1 drugs.
The fact that the overwhelming majority of GLP-1 administration is parenteral, combined with the preference of both drug sponsors and patients for easy-to-use device formats (typically injection pens and/or autoinjectors), presents a massive opportunity for drug delivery system manufacturers. However, on the flip side, it also introduces a significant sustainability challenge, as the current landscape is dominated by disposable devices. The projected increase in patient numbers will only magnify this issue.
“ADVANCES IN DRUG ENGINEERING MAY ENABLE EVEN LONGER DOSING INTERVALS, WITH A SMALL BUT GROWING PERCENTAGE OF CLINICAL CANDIDATES AIMING FOR MONTHLY OR LONGER
INJECTION CYCLES.”
The simplest step towards mitigation is shifting from single-dose autoinjector formats to multidose pens where preservability allows. However, beyond that, solutions become less straightforward. Advances in drug engineering may enable even longer dosing intervals, with a small but growing percentage of clinical candidates aiming for monthly or longer injection cycles. If successful, these options could reduce the aggregate number of devices required, which has led some industry stakeholders to consider them a sustainability lever. That said, the true impact on plastics consumption is debatable, as a single-dose monthly injection may still require similar amounts of materials as a multidose weekly injection device.
While monthly GLP-1 formulations may become commercially available soon in higher-income regions, other geographies – especially those with high expected biosimilar penetration – may continue relying on weekly-dosed drugs for the foreseeable future. This means that theentire GLP-1 supply chain, particularly drug delivery system manufacturers, must take a proactive approach in developing more sustainable device solutions.
A DATA-DRIVEN APPROACH TO SUSTAINABILITY
Ypsomed has taken up this challenge with its Net Zero Program – a structured approach to reducing the carbon footprint of self-injection devices while maintaining ease of use and full compliance. As part of its long-term sustainability commitment, Ypsomed has joined the Science Based Targets initiative (SBTi), targeting net zero emissions across its entire value chain by 2040. This ensures that its sustainability efforts follow scientifically validated reduction pathways and deliver measurable impact.
As part of the Net Zero Program, Ypsomed conducts lifecycle assessments in accordance with ISO 14040/44 standards, using an established methodology verified by independent third parties. These assessments provide detailed insights into the carbon footprint of Ypsomed’s products, identifying key areas for optimisation and immediate opportunities for improvement. With this data-driven approach, sustainability measures are targeted and effective.
ADVANCED MATERIALS FOR A LOWER FOOTPRINT
While new delivery solutions are being explored, many of today’s GLP-1 therapies still rely on established autoinjector platforms. As the market continues its rapid expansion, ensuring that these widely used devices are as sustainable as possible is a key priority. With single-use systems remaining a dominant format, reducing their environmental footprint is critical to ensuring long-term viability.
To address this, Ypsomed has integrated its Net Zero Program into its proven self-injection platforms. The YpsoMate 1 and 2.25 mL autoinjectors – already in use for various biologics, including GLP-1s – have been redesigned to achieve a lower carbon footprint without requiring any changes to drug formulation, delivery protocols or device.
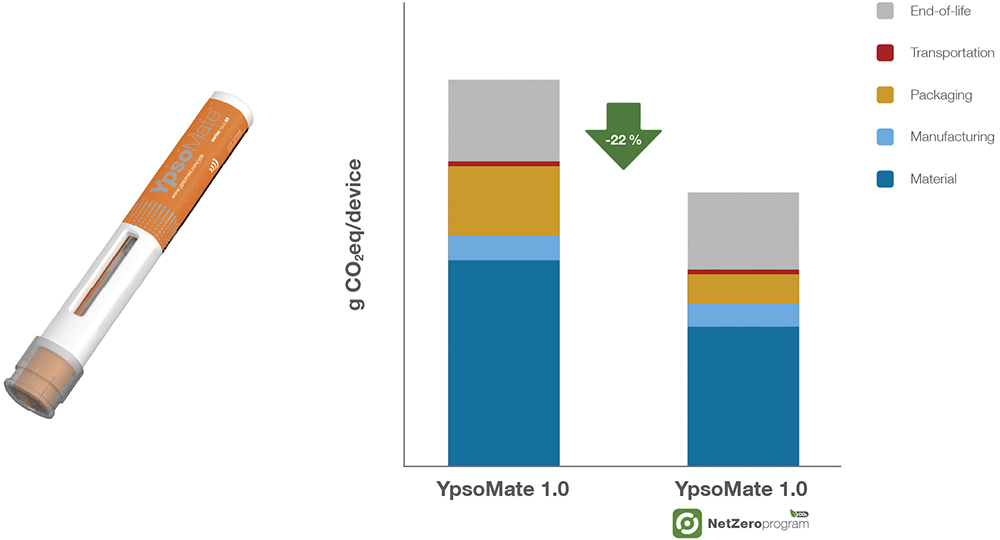
Figure 2: CO2 reduction impact of the Net Zero Program – comparison of the carbon footprint (g CO2eq/device) between the standard YpsoMate and YpsoMate within the Net Zero Program, highlighting a 22% carbon footprint reduction across materials, production, packaging, transportation and end-of-life (EoL) processes.
A key driver behind the 22% carbon footprint reduction of the YpsoMate 1 mL autoinjector is the shift to bio-based materials – chemically identical to conventional plastics, sustainably sourced and requiring no additional regulatory approvals or manufacturing process adjustments (Figure 2). Importantly, this transition does not necessitate any changes in formulation, stability or device function. This sustainability shift is further reinforced by ISCC+ certification, ensuring compliance with rigorous industry standards. The same approach has been applied to the YpsoMate 2.25 mL autoinjector, achieving an even greater carbon footprint reduction of 31% (Figure 3).
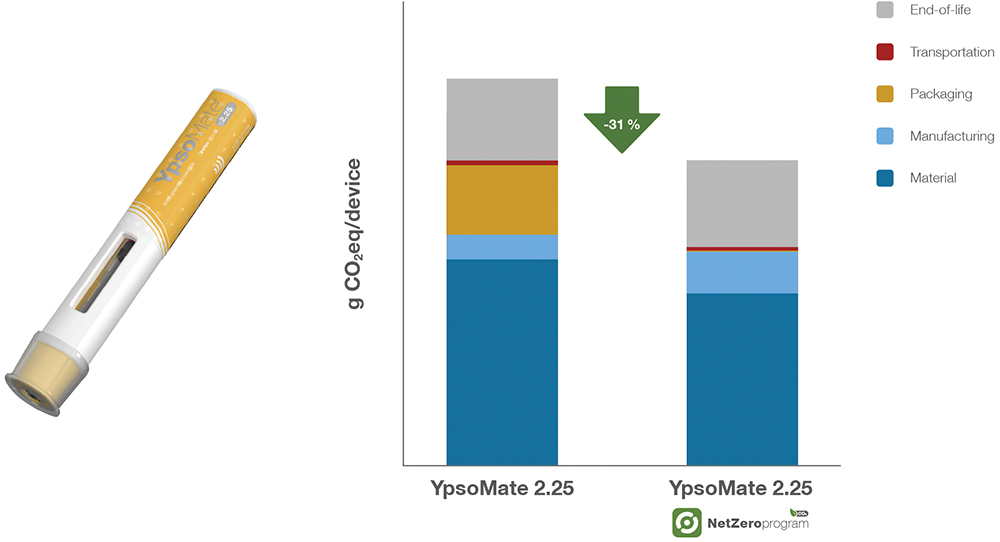
Figure 3: CO2 emission reduction of the Net Zero Program – a 31% reduction in carbon footprint (g CO2eq/device) for the YpsoMate 2.25 mL autoinjector when integrated into the Net Zero Program, demonstrating lower emissions across materials, manufacturing, packaging, transportation and EoL processes.
PLUG-AND-PLAY SUSTAINABILITY FOR PHARMA SUPPLY CHAINS
Beyond material innovations, the net zero sustainability approach extends across the entire supply chain. Since the bio-based materials used in Ypsomed’s devices retain the same chemical properties as conventionalplastics, the transition requires no additional regulatory approvals or manufacturing modifications – a key advantage already outlined. This allows pharmaceutical companies to reduce their environmental footprint with minimal operational impact, a growing priority as sustainability regulations become increasingly stringent.
In addition to making products more sustainable through material selection, Ypsomed’s Net Zero Program also incorporates optimised packaging solutions, which prioritise the use of recycled materials for trays and a shift to wooden pallets, minimising the overall carbon footprint while ensuring that pharmaceutical logistics maintain the highest quality and safety standards.
UNOPEN: SUPPORTING THE EXPANSION OF GLP-1 IN MULTIDOSE FORMATS
While single-dose autoinjectors remain dominant in GLP-1 delivery, multidose pen injectors also play a critical role, particularly in formulations where preservability allows. As demand for GLP-1 therapies expands, balancing injection frequency, sustainability and drug stability is becoming increasingly important. In this context, multidose devices can help reduce material consumption while maintaining flexibility in treatment regimens.
To support this transition, Ypsomed has applied its net zero sustainability principles to multidose injection systems as well. The UnoPen, a prefilled pen injector, integrates ISCC+ certified bio-based materials to achieve a 32% CO2 reduction – offering a more sustainable alternative for therapies administered in a multidose format (Figure 4). Like YpsoMate, this sustainability shift requires no regulatory changes, making it easy for pharmaceutical companies to integrate into existing treatment protocols.
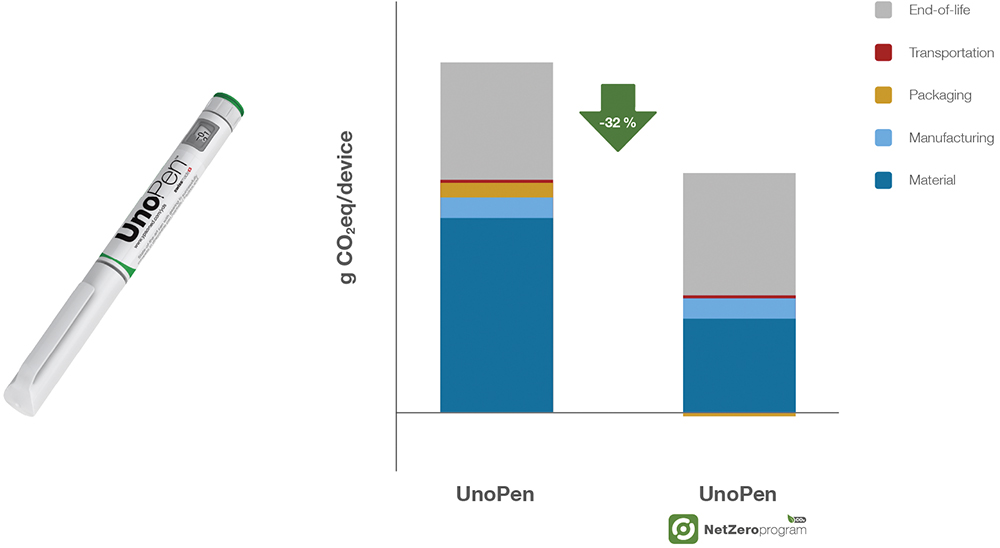
Figure 4: CO2 emission reduction for UnoPen in the Net Zero Program – comparison of the carbon footprint (g CO2eq/device) between the standard UnoPen and UnoPen within the Net Zero Program, showing a 32% reduction in carbon footprint (g CO2eq/device) across materials, manufacturing, packaging, transportation and EoL processes.
ECODESIGN IN HIGH-VOLUME DRUG DELIVERY: YPSOMATE 5.5
As GLP-1 therapies evolve and higher-dose formulations are being explored to further reduce injection frequency, the demand for higher-volume autoinjectors is increasing to support these advancements and meet drug delivery requirements. Beyond standard-dose autoinjectors, Ypsomed has also applied its structured sustainability principles to larger-volume delivery. The YpsoMate 5.5, designed for high-volume biologics, follows ecodesign principles embedded within Ypsomed’s development process. By ensuring that sustainability considerations are integrated from the earliest design stages, including material selection guided by the Ecodesign Guideline, the company can offer a CO2-reduced variant of YpsoMate 5.5 from launch (Figure 5).
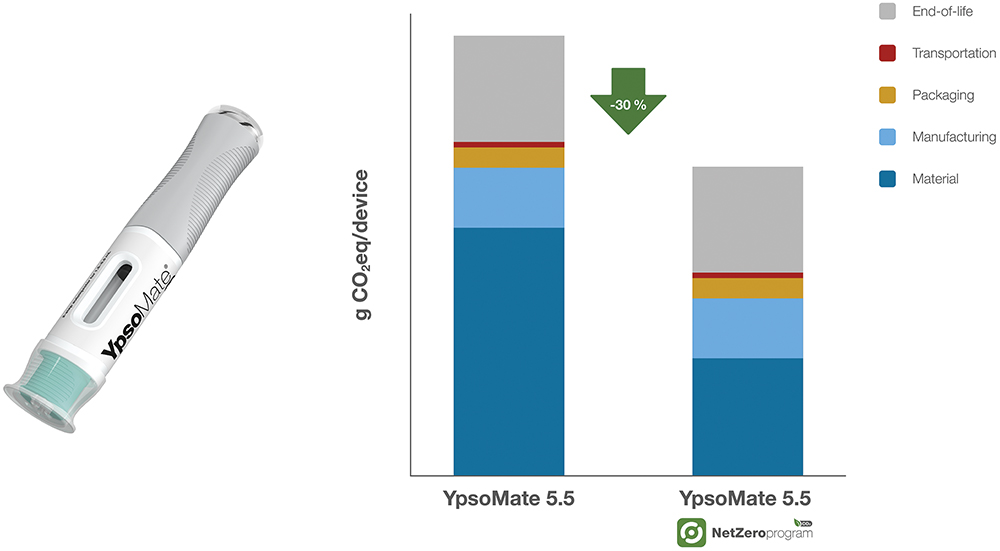
Figure 5: CO2 emission reduction for YpsoMate 5.5 mL in the Net Zero Program – comparison of the carbon footprint (g CO2eq/device) between the standard YpsoMate 5.5 mL and YpsoMate 5.5 mL within the Net Zero Program, illustrating significant reductions across materials, manufacturing, packaging, transportation and EoL processes.
Despite its larger size, the YpsoMate 5.5 remains aligned with Ypsomed’s sustainability efforts, allowing pharmaceutical companies to adopt a more environmentally responsible solution without compromising usability or performance. This approach highlights how innovation and sustainability can go hand in hand, ensuring that even as drug formulations evolve, delivery systems keep pace with sustainability goals.
“YPSOMED OFFERS TWO HIGH-QUALITY REUSABLE PEN INJECTORS, DESIGNED TO EXTEND PRODUCT LIFESPANS AND REDUCE MATERIAL CONSUMPTION OVER TIME.”
BEYOND SINGLE-USE: THE ROLE OF REUSABLE PEN INJECTORS
While the Net Zero Program significantly reduces the environmental impact of single-use devices, reusable solutions provide an additional pathway towards sustainability. Ypsomed offers two high-quality reusable pen injectors, designed to extend product lifespans and reduce material consumption over time. For GLP-1 therapies, switching to a reusable device can lead to a waste reduction of up to 96% and a CO2 reduction of 94%, further amplifying the environmental benefits (Ypsomed, internal data on file, 2024; Figure 6).
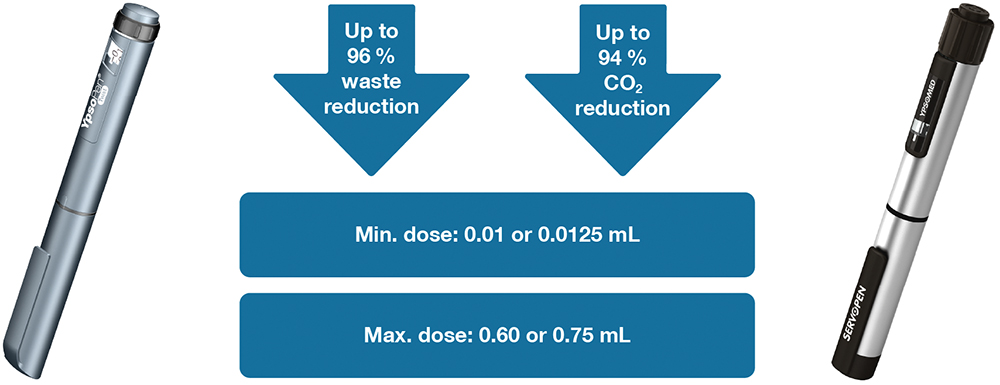
Figure 6: YpsoPen and ServoPen – Ypsomed’s reusable pen injectors designed for sustainability, offering up to 96% waste reduction and 94% CO2 reduction. These devices support precise dosing, with a minimum dose of 0.01 or 0.0125 mL and a maximum dose of 0.6 or 0.75 mL, making them suitable for long-term GLP-1 therapies (Ypsomed, internal data on file, 2024).
The ServoPen is a premium reusable pen injector, featuring a metal housing for enhanced durability and a high-end user experience. With an in-use time of three years, this pen contributes to significant waste reduction by minimising the need for disposable components. With GLP-1 drugs being prescribed as long-term therapies, a durable, reusable device can significantly reduce plastic waste and material consumption. This makes the ServoPen a sustainable choice for ongoing GLP-1 treatment, particularly in regions where cost effectiveness and long-term adherence are priorities.
For more cost-sensitive applications, the YpsoPen provides another reusable solution, designed for ease of use while still delivering the sustainability benefits of a multi-use system. Made from lightweight yet durable plastic, it helps expand access to GLP-1 therapies, balancing affordability with reduced environmental impact.
A PRAGMATIC PATH TO A GREENER FUTURE
The GLP-1 era is unfolding at a rapid pace, reshaping treatment landscapes for obesity and metabolic diseases. But with this progress comes responsibility – ensuring that the delivery of these breakthrough therapies aligns with the sustainability imperatives of the future. The growing demand for self-injection devices cannot be met with traditional solutions alone; the industry must evolve, adopting forward-thinking approaches that reduce environmental impact without compromising patient care.
Ypsomed stands at the forefront of this transformation. Through its Net Zero Program, the company is not only providing CO2-reduced alternatives but also setting a new standard for sustainability in drug delivery. With a firm commitment to scientifically validated impact reduction, innovative material use and seamless supply chain integration, Ypsomed is empowering pharmaceutical companies to make meaningful strides toward greener drug administration.
The future of GLP-1 drug delivery is not just about efficacy – it is about responsibility. As the industry scales, Ypsomed is ensuring that sustainability keeps pace, proving that innovation and environmental stewardship can go hand in hand. The path to net zero in self-injection devices has been laid, and Ypsomed is leading the way.
REFERENCES
- Eng J et al, “Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas”. J Biol Chem, 1992, Vol 267, pp 7402–7405.
- Byetta Injection approval. US FDA, April 28, 2005.
- Zheng Z et al, “Glucagon-like peptide-1 receptor: mechanisms and advances in therapy”. Sig Transduct Target Ther, 2024, Vol 9, (1), p 234.
- Annual Report, Novo Nordisk, 2024.
- “World of Obesity Atlas”, World Obesity, 2023.
- Hui H et al, “The short half-life of glucagon-like peptide-1 in plasma does not reflect its long-lasting beneficial effects”. Eur J Endocrinol, 2002, Vol 146 (6), pp 863–9.

