To Issue 172
Citation: Saaler-Reinhardt S, Lennerz A, Mond M, Stockton M, “Greater Formulation Freedom with a Novel Autoinjector Platform that Extends Volume and Viscosity Limits”. ONdrugDelivery, Issue 172 (May 2025), pp 68–73.
Prof Sigrid Saaler-Reinhardt, Dr Andrea Lennerz, Moritz Mond and Marie Stockton, discuss how the Gx Inbeneo® autoinjector overcomes the limitations of prefilled syringe-based autoinjectors to provide effective, safe administration of large-volume, high-viscosity formulations.
The market growth of approved biotherapeutics, particularly monoclonal antibodies (mAbs), has driven demand for self-injection devices, such as autoinjectors.1 These devices, designed for subcutaneous (SC) or intramuscular (IM) injection, encountered challenges when required to deliver protein-based formulations. Limited delivery volume of standard autoinjectors can require higher concentrations to achieve therapeutic efficacy resulting in higher viscosity, or the need to dilute, which increases volume.2
In addition to mAbs with immediate- release profiles, there is a growing development pipeline of long-acting injectables with advanced delivery systems, such as nano-suspensions, emulsions, liposomes and microspheres,3,4 some of which can exhibit very high viscosities.5 Administering these complex formulations effectively and comfortably necessitates specialised delivery devices.6
The Gx Inbeneo® autoinjector, developed by Gerresheimer and Midas Pharma, addresses these challenges through a novel pre-pressurised cartridge-based design with a double-ended needle system.7 This enables delivery of larger volumes and higher viscosities while also supporting patient adherence through user-centric design and tolerable injection times. The Gx Inbeneo® overcomes the limitations of prefilled syringe (PFS)-based autoinjectors in terms of viscosity, volume and injection time. Formulations scientists thus have greater flexibility when developing biologic drug products for SC self-administration.
CHALLENGES IN FORMULATING BIOLOGICS FOR SC ADMINISTRATION
High Concentration and Viscosity Considerations for Stability and Manufacture
Sensitive biotherapeutics, such as mAbs, are vulnerable to aggregation, which may lead to a loss of biologic functionality. Transitioning from intravenous (IV) to SC self-administration often requires higher concentrations to achieve the necessary therapeutic dose within a manageable injection volume, leading to higher viscosity.8 This complicates delivery via standard syringes formats, as higher forces and larger needle gauges are needed to achieve acceptable injection times for patients.
Water-based immunoglobulin G (IgG) solutions, which include mAbs, are generally relatively low viscosity and exhibit Newtonian behaviour up to approximately 230 mg/mL. However, above 300 mg/mL, viscosity increases dramatically due to intermolecular interactions, such as self-association between molecules, causing protein aggregation and potentially resulting in non-Newtonian shear-thinning behaviour.9 This can lead to negative consequences, such as functional inactivation.10
Although mAbs are IgG molecules, they vary in behaviour according to their physico-chemical properties.11 Maintaining protein stability and preventing aggregation in mAbs during storage and transportation often requires adding excipients or adjusting salt composition and pH,8 which can further increase viscosity. Highly concentrated viscous formulations also complicate manufacturing, particularly during filtration and filling.12,13
Most marketed mAbs are in the range of 100–200 mg/mL, generally maintaining viscosities under 30 cP, though some reach 40–100 cP.11,14 Above 200 mg/mL, viscosity can reach up to 200 cP, and may exhibit non-Newtonian behaviour.9
High-viscosity formulations require either longer injection times or larger needles, negatively impacting patient comfort and ease of administration, which may affect patient adherence.15 Maintaining stability and optimal viscosity remains a major challenge in SC biologic formulations.
Self-Administration Challenges
SC self-administration is increasingly preferred, as it facilitates greater patient independence and reduces the burden on healthcare systems. Autoinjectors offer patient usability and safety advantages, such as needlestick protection, but they also present challenges:
- Patient Comfort: High-injection forces can cause pain, necessitating finer needles.16
- Adherence: Extended injection times and complex handling may reduce adherence, particularly for patients with conditions that impact dexterity, such as rheumatoid arthritis.
- Efficacy: Ensuring complete dosing within an acceptable timeframe is crucial for therapeutic effectiveness.17
THE LIMITATIONS OF PFS-BASED AUTOINJECTORS
Most conventional autoinjectors that rely on a PFS face several limitations when handling high-viscosity and large-volume formulations.
Such autoinjectors employ a staked-needle PFS combined with a compressed spring held in place with a locking mechanism. This must then be reliably released at the moment of injection, which can cause high-impact forces18 that place stress on components. The higher spring force required to deliver drugs in large volumes or high viscosity can be particularly problematic and may lead to misfiring.19
Due to these design limitations, PFS-based autoinjectors can typically handle up to a maximum volume of 2.25 mL. The combination of spring force and needle gauge also restricts their ability to deliver high-viscosity formulations without unacceptably long injection times.
Another disadvantage of a staked-needle PFS when handling viscous biologic drug products or suspensions is the risk of clogging during storage. Aggregation caused by migration of silicone oil droplets is another challenge that is significantly reduced by employing a cartridge with baked-on siliconisation.20
“THE GERRESHEIMER GX INBENEO® AUTOINJECTOR PLATFORM WAS SPECIFICALLY DEVELOPED TO OVERCOME THE BOUNDARIES OF TRADITIONAL PFS-BASED SYSTEMS, ENABLING THE DELIVERY OF VOLUME UP TO 3 ML AND VERY HIGH-VISCOSITY FORMULATIONS.”
OVERCOMING BOUNDARIES WITH A CARTRIDGE-BASED, DOUBLE-NEEDLE DESIGN
The Gerresheimer Gx Inbeneo® autoinjector platform was specifically developed to overcome the boundaries of traditional PFS-based systems, enabling delivery of volumes up to 3 mL and very high-viscosity formulations.
Cartridge-Based and Pre-Pressurised
The use of a glass cartridge as the primary container in the Gx Inbeneo® facilitates many of its advantages. The novel design is pre-pressurised and employs the cartridge as the force barrier, which eliminates the need for an additional spring-locking mechanism. Stronger springs can therefore be used – enabling the delivery of higher viscosities and avoiding a pressure spike – for a steady delivery profile. An additional benefit of the pre-pressurised design is the reduction or elimination of a gas bubble according to Henry’s Law, which may otherwise cause aggregation in sensitive drugs.21 Functional and usability testing of the Gx Inbeneo®, which confirms efficacy and patient acceptance, is outlined in an ONdrugDelivery article from October 2024.22
Platform Provides Freedom to Choose Needle Options
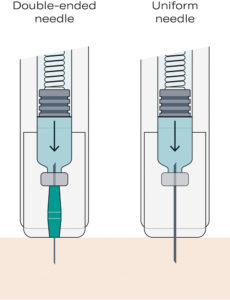
Figure 1: The double-ended needle design optimises flow dynamics due to different diameters of the needle for piercing the patient’s skin and septum.
As the Gx Inbeneo® needle is separated from the primary cartridge until the moment of injection, the risk of the biologic drug molecules or excipients clogging the needle during storage is eliminated. The Gx Inbeneo® platform concept provides pharmaceutical customers with the freedom to not only select the appropriate cartridge size and spring force but also the appropriate needle for their specific drug formulation. Customisation of the needle can include outer needle diameters, inner diameters, thin/ultra-thin walls and specific tip geometries. However, the optimal choice is the Gx Inbeneo®’s unique double-ended needle system (Figure 1).
Advantages of a Double-Ended Needle
In typical autoinjectors, selecting a needle gauge involves a trade-off between flow rate and patient comfort. To accelerate drug delivery, a larger diameter needle can be chosen, but it may increase injection pain. Conversely, a thinner needle is gentler for the patient but can lead to slow injection times or higher injection forces. The Gx Inbeneo® helps to resolve this conflict by employing a double-ended needle system with two different needle gauges in one integrated assembly.
On the cartridge side of the needle, a larger internal diameter is used to pierce the cartridge septum, enabling higher flow rates for viscous solutions and significantly reducing flow resistance. This allows the drug to pass quickly through the needle even when dealing with elevated viscosities or larger injection volume. The needle diameter on the patient end is deliberately smaller to minimise discomfort.
The double-ended needle concept directly addresses one of the biggest challenges in delivering large volumes or highly viscous formulations subcutaneously by reducing the compromise between flow rate and patient comfort.
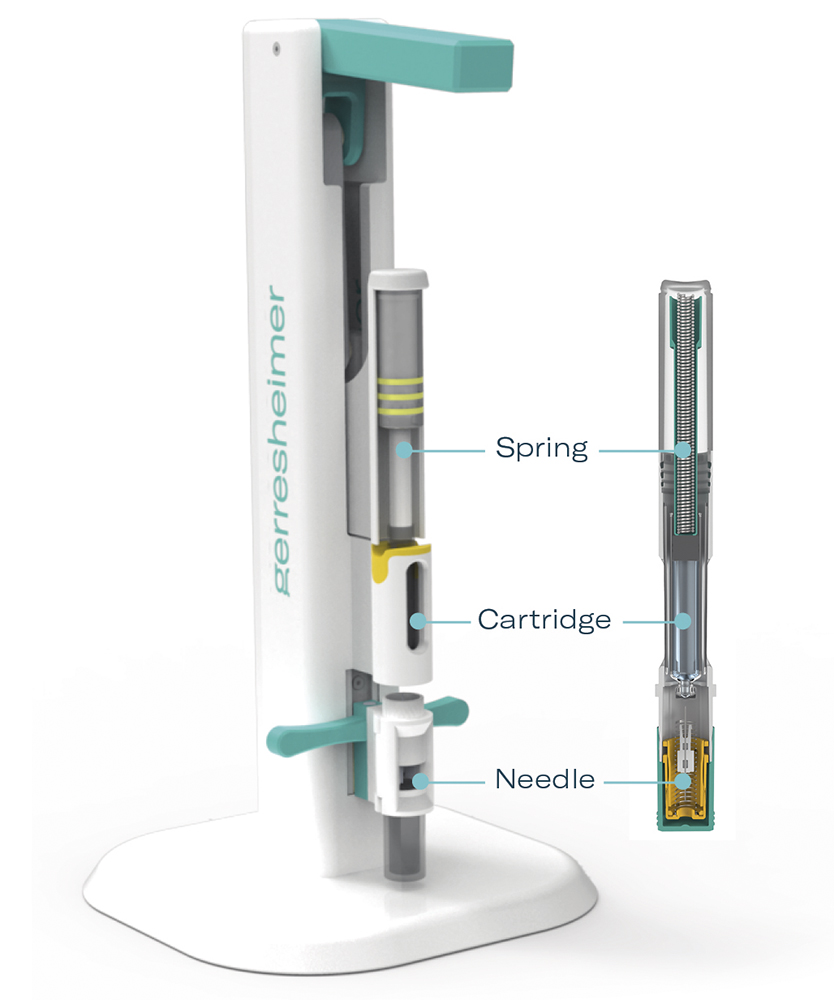
Figure 2: The Gx Inbeneo® platform simulator correlates to the autoinjector platform and enables a tailored assembly of spring force, needle size and ISO-cartridge volume for testing.
TESTING THE LIMITS
Gerresheimer and Midas Pharma recently conducted comprehensive testing of the Gx Inbeneo® autoinjector to evaluate its capability to deliver highly viscous drug formulations and large volumes. Testing was carried out with the Gx Inbeneo® platform simulator, which mimics the autoinjector platform, enabling different combinations of spring force, needle gauge and volume. The simulator can, therefore, provide real-life results, substantiating theoretical calculations using Hagen-Poiseuille’s law. Formulation scientists can use the simulator to test injection times for their specific formulation at different stages of development (Figure 2).
The test series was conducted with both 1.5 and 3.0 mL ISO glass cartridges, paired with 45 and 90 N spring power units, respectively. In addition, 25G and 27G double-ended needles were tested, as well as a 25G uniform needle for comparison. Glycerol and distilled water were mixed in varying viscosities according to weight percentages calculated using an established viscosity calculation script. The cartridges were filled and sealed by carefully mounting the plunger with a thin steel implement to minimise air entrapment. Strict protocols were followed, with every measurement repeated five times at a temperature of approximately 21°C. The described results give the mean value of each test.
Large-Volume Delivery
To evaluate the full capacity of the Gx Inbeneo®, 3 mL ISO cartridges were filled with glycerol solutions of varying viscosities and combined with 25G and 27G double-ended needles. Several key results demonstrated the success of the design.
Using a 27G double-ended needle, a viscosity of 20 cP could be expelled in less than 15 seconds, and up to 40 cP in approximately 26 seconds (interpolated). Midas Pharma / Gerresheimer Injection Devices Figure 1: The double-ended needle design optimises flow dynamics due to different diameters of the needle for piercing the patient’s skin and septum. Figure 2: The Gx Inbeneo® platform simulator correlates to the autoinjector platform and enables a tailored assembly of spring force, needle size and ISO-cartridge volume for testing. By increasing the needle diameter to 25G, 75 cP could be expelled in 10 seconds and viscosity exceeding 160 cP could be delivered within 20 seconds (Figure 3).
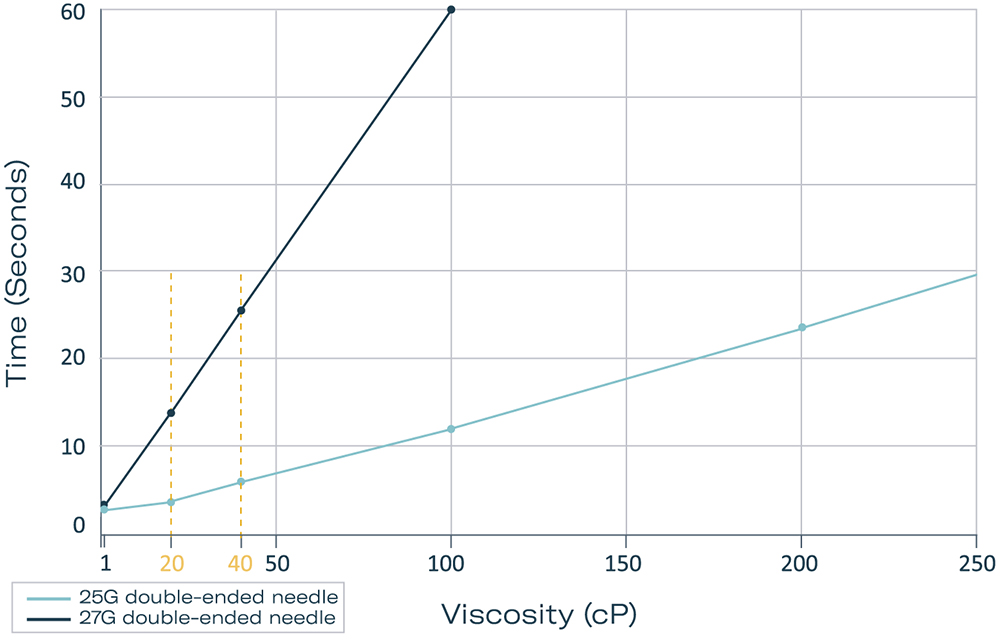
Figure 3: Comparison of injection times with 25 and 27 G double-ended needles for 3 mL of liquid of different viscosities.
Proven Advantage of a Double Needle
Direct comparison between standard and double-ended needles revealed a significant difference in injection time. The double-ended needle expelled a liquid of 20 cP approximately 10 seconds faster than a uniform needle. With the increase in viscosity, this improvement became ever more apparent, with a five-fold decrease in injection time recorded with the double-ended needle compared with a uniform needle under the same conditions. When reaching a viscosity of 200 cP, the double-ended needle was able to expel 3 mL in approximately 23 seconds. Even with very high viscosities of 500 cP and a volume of 1 mL, the delivery time remained under 30 seconds with the double-ended needle (Figure 4).
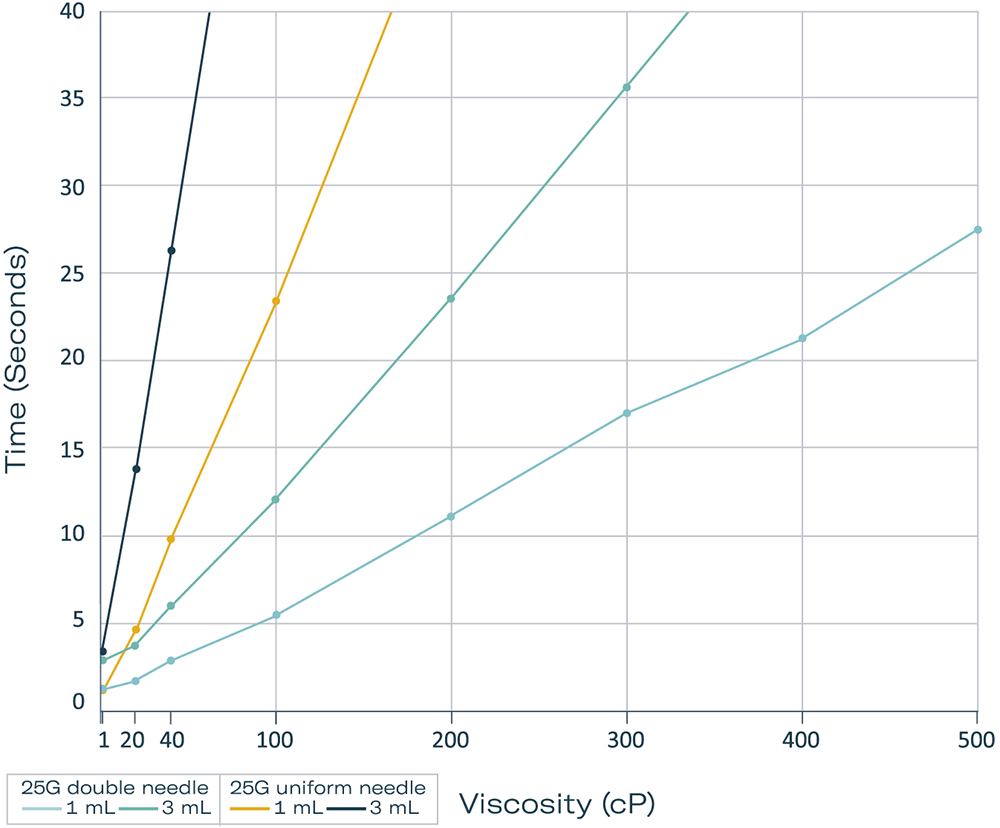
Figure 4: Comparison of injection times with 25 G uniform and double-ended needles and volumes of 1.0 and 3 mL.
“ANOTHER INTERESTING FINDING, PARTICULARLY WHEN CONSIDERING PATIENT COMFORT, IS THAT THE 27G DOUBLE-ENDED NEEDLE SHOWED COMPARABLE RESULTS WITH A 25G
UNIFORM NEEDLE.”
Another interesting finding, particularly when considering patient comfort, is that the 27G double-ended needle showed comparable results with a 25G uniform needle (Figures 3 and 4).
Efficacy at Very High Viscosity
At extremely high viscosity and lower volume, the Gx Inbeneo® also proved its efficacy. At viscosities >1200 cP, 0.5 mL could be delivered in less than 30 seconds. When the volume increased to 1 mL, viscosity as high as 500 cP was still achieved in less than 30 seconds (Figure 5).
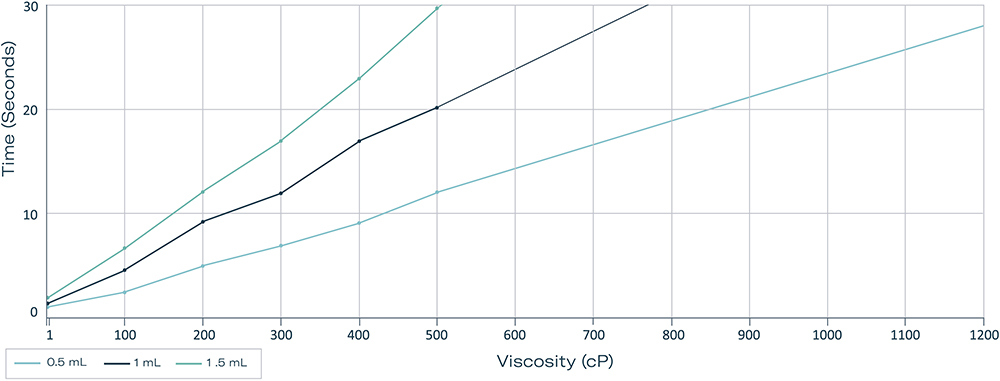
Figure 5: Measurement of maximum viscosities delivered with a 25 G double-ended needle in 30 seconds at volumes of 0.5, 1 and 1.5 mL in a 1.5 mL ISO cartridge.
The results demonstrate the superior performance of the Gx Inbeneo® over traditional syringe-based autoinjectors, which can handle less volume and often struggle to deliver above 50 cP within a tolerable injection time.23,24
ADVANTAGES FOR FORMULATION SCIENTISTS
For formulation scientists, the Gx Inbeneo® platform provides significant advantages:
- Greater Flexibility in Drug Concentration: The ability to deliver higher viscosities subcutaneously without excessive dilution means biologics, as well as long-acting complex formulations, can be formulated at more optimal concentrations without impacting efficacy.
- Reduced Need for Excipients: Avoiding unnecessary excipients that lower viscosity can enhance stability and reduce potential immunogenicity risks.8
- Larger Volumes Made Possible: Injection times are a critical factor. The tests demonstrate that Gx Inbeneo® can deliver larger doses without longer injection times compromising patient comfort.
- Compatibility with Next-Generation Biologics: Many pipeline biologics, including bispecific antibodies and antibody-drug conjugates, have inherently higher viscosities due to their inclination to form aggregates. The Gx Inbeneo® accommodates these formulations more effectively than traditional autoinjectors.
PATIENT-CENTRIC DESIGN SUPPORTS ADHERENCE
The benefits of the Gx Inbeneo® platform design not only include greater formulation flexibility but it is also user-centric – something that is at the core of all product development at Gerresheimer. Faster injection times for high-viscosity formulations enable a broader range of high-viscosity drugs to be administered in a clinically acceptable and tolerable injection time. Reduced injection force due to optimised needle dynamics improves usability and may also reduce pain. A unique transparent top casing allows the patient or healthcare professional to visually track injection progress, and the needle remains hidden before and after injection, reducing needle phobia and risk of needlestick injury. Such features enhance user experience and safety and may aid patient adherence.
CONCLUSION
The Gx Inbeneo® platform represents a breakthrough in SC biologic drug delivery by enabling the safe, effective administration of large-volume, high-viscosity formulations using an autoinjector. Its cartridge-based design provides formulation scientists with the flexibility to develop stable and effective biologics while ensuring patient comfort and adherence. The innovative design has not only demonstrated efficacy and user-centricity through extensive testing but was also awarded a prestigious Red Dot Design Concept award in October 2024.
As the market for biologics continues to expand, innovative drug delivery systems, such as the Gx Inbeneo®, will play a crucial role in helping to achieve treatment outcomes that, ultimately, enhance patient quality of life.
ACKNOWLEDGEMENT
Special thanks to Lorella Heigel, Masters student in Medical and Pharmaceutical Biotechnology, who conducted the testing.
REFERENCES
- Guo J et al, “A review of recent FDA-approved biologic-device combination products”. J Pharm Sci, 2024, Vol 113, pp 866–879.
- Bittner B et al, “Subcutaneous administration of biotherapeutics: an overview of current challenges and opportunities”. BioDrugs, 2018, Vol 32(5), pp 425–440.
- Panchal K et al, “An expanding horizon of complex injectables products: development and regulatory considerations”. Drug Deliv Transl Res, 2023, Vol 13(2), pp 433–472.
- Wilhelmy C et al, “Polysarcosine-functionalized mRNA lipid nanoparticles tailored for immunotherapy”. Pharmaceutics, 2023, Vol 14(8), art 2068.
- Muthu MS et al, “PLGA nanoparticle formulations of risperidone: preparation and neuropharmacological evaluation”. Nanomedicine, 2009, Vol 5(3), pp 323–333.
- Zang Q et al, “Delivery considerations of highly viscous polymeric fluids mimicking concentrated biopharmaceuticals: assessment of injectability via measurement of total work done ‘WT’”. AAPS PharmSciTech, 2018, Vol 19(4), pp 1520–1528.
- Primavessy et al, “Gx Inbeneo® – Developing an autoinjector that responds to the challenges of biologics”. ONdrugDelivery, Issue 152 (Oct 2023), pp 32–36.
- Desai M et al, “Monoclonal antibody and protein therapeutic formulations for subcutaneous delivery: high – concentration, low volume vs. low concentration, high volume”. MAbs, 2023, Vol 15(1), 285277.
- Bruckbuchler V et al, “Rheological and syringeability properties of highly concentrated human polyclonal immunoglobulin solutions”. Europ J Pharm Biopharm, 2010, Vol 76(3), pp 251–356.
- Apgar JR et al, “Modeling and mitigation of high-concentration antibody viscosity through structure-based computer-aided protein design”. PLoS ONE, 2020, Vol 15(5), art e0232713.
- Haeri HH et al, “Concentration effects in the interaction of monoclonal antibodies (mAbs) with their immediate environment characterizes by EPR spectroscopy”. Molecules, 2019, Vol 24(14), art 2528.
- Shire SJ et al, “Challenges in the development of high protein concentration formulations”. J Pharm Sci, 2004, Vol 93(6), pp 1390–1402.
- Garidel P et al, “High-concentration protein formulations: How high is high?” Eur J Pharm Biopharm, 2017, Vol 119, pp 353–360.
- Wang SS et al, “US FDA-approved therapeutics antibodies with high-concentration formulation: summaries and perspectives”. Antib Theraps, 2021, Vol 4(4), pp 262–272.
- Stoner KL et al, “Intravenous versus subcutaneous drug administration. Which do patients prefer? A systematic review”. Patient, 2014, Vol 8, pp 145–153.
- Pager A et al, “How shorter needles with thinner walls are set to improve the injection experience in chronic care”. OnDrugDelivery, Issue 107 (May 2020), pp 37–42.
- Mathaes R et al, “Subcutaneous injection volume of biopharmaceuticals – pushing the boundaries”. J Pharm Sci, 2016, Vol 105(8), pp 2255–2259.
- Veilleux JC, Shepherd JE, “Pressure and stress transients in autoinjector devices”. Drug Deliv Transl Res, 2018, Vol 8(5), pp 1238–1253.
- “Simponi (golimumab): important changes to the injection instruction for the Smart-Ject Pre-filled Pen”. Direct Healthcare Professional Communication, MSD, Aug 2023.
- Murphy MI et al, “Effect of Various Silicone Oil and Tungsten Levels on the Stability of a Monoclonal Antibody in Nine Commercially Available Pre-filled Syringes”. J Pharm Sci, 2023, Vol 112(6), pp 1586–1594.
- Leiske DL et al, “A method to measure protein unfolding at an air-liquid interface”. Langmuir, 2016, Vol 32(39), pp 9930–9937.
- Seddighi F et al, “Comprehensive testing proves design concept of a novel autoinjector platform”. ONdrugDelivery, Issue 166 (Oct 2024), pp 47–50.
- Schneider A et al, “Hold the device against the skin: the impact of injection duration on user´s force for handheld autoinjectors”. Expert Opin Drug Deliv, 2020, Vol 17(2), pp 225–236.
- Badkar AV et al, “Subcutaneous delivery of high-dose/volume biologics: Current status and prospect for future advancements”. Drug Des Devel Ther, 2021, Vol 15, pp 159–170.

