To Issue 174
Citation: Kelly A, “Navigating Semiconductor Lifecycle Challenges in Medtech: a Strategic Shift Towards ASICs”. ONdrugDelivery, Issue 174 (Jun 2025), pp 22–26.
Andy Kelly considers the evolution of semiconductor technology and the impact of the release of new and upgraded components. This article discusses how strategic decisions during the early stages of product design can determine the future viability of a medical device and considers the cost savings offered by application-specific integrated circuits through a reduction in the number of components required.
Semiconductor technology is evolving at a rapid pace,1 providing ever-increasing levels of complexity, performance and efficiency for consumer and industrial products. While these advances benefit all product categories, they also present a unique dilemma for medical device developers. The frequent release of new and upgraded semiconductor components increases the risk of their predecessors becoming obsolete. This is an especially critical issue for implantable medical devices, which typically involve long cycles of development and regulatory approvals, as well as long product lifetimes.
“IN CONSUMER-DRIVEN MARKETS, SEMICONDUCTOR TECHNOLOGY CHANGES RAPIDLY TO MEET PERFORMANCE, EFFICIENCY AND COST TARGETS. NEW FABRICATION TECHNOLOGIES AND ENHANCED CIRCUIT DESIGNS ARE INTRODUCED REGULARLY.”
SEMICONDUCTOR LIFECYCLE CHALLENGES IN MEDTECH
In consumer-driven markets, semiconductor technology changes rapidly to meet performance, efficiency and cost targets. New fabrication technologies and enhanced circuit designs are introduced regularly. Due to these technological advancements, shifting market demands and manufacturers prioritising high-volume, short-lifecycle applications, standard semiconductor components, such as power management integrated circuits (ICs), sensor ICs, driver ICs and microcontrollers often become obsolete within a few years of their introduction.
As implantable medical devices require extensive, multi-year regulatory approval processes and are expected to remain in the market for a decade or more, the potential for component obsolescence is particularly challenging. When a key component becomes unavailable, medical device manufacturers are forced to choose between an expensive lifetime buy (purchase of enough components to support the entire product lifecycle) or a redesign of the device electronics – both of which present high costs and risks.
DESIGNING FOR LONGEVITY AND COMPLIANCE
To navigate these high-stakes challenges, medical device manufacturers should take a proactive, forward-looking approach to design and development – one that prioritises component longevity and aligns with performance requirements and long-term regulatory cycles. Strategic decisions made during the initial design phases often determine the future viability of a medical device. Selecting components with short lifecycle expectations introduces unnecessary risks in the form of obsolescence and potential supply disruptions. Conversely, overinvesting in unproven technologies may not necessarily pay off if future support vanishes due to low adoption, shifts in market demand or discontinuation of the product line by the manufacturer.
This is where expert semiconductor product development partners play a critical role. Design teams with a deep understanding of semiconductor roadmaps, medical regulatory requirements and circuit design partitioning can help companies make smart choices early, avoiding costly disruptions later (Figure 1).
Figure 1: Design teams can help companies to make smart choices early, avoiding costly disruptions later.
THE VALUE OF ASICs IN MEDICAL DEVICE DESIGN
Application-specific integrated circuits (ASICs) offer a compelling solution for many medical device developers. By integrating multiple analogue and digital functions into a single, application-specific chip, multiple standard components can be eliminated from the device. As a result, there are fewer suppliers in the supply chain, and components at risk of obsolescence are reduced – thereby making the continuity of supply more secure. Unlike off-the-shelf components designed for high-volume, short-lifecycle markets, ASICs are engineered for specific applications and exclusively sold to specific device developers. This specificity gives manufacturers greater control over design and lifecycle planning, reducing their exposure to industry-wide fabrication changes, shifting consumer trends or other common obsolescence factors.
“TO REDUCE ASIC OBSOLESCENCE RISK, THE UNDERLYING SILICON TECHNOLOGIES USED TO BUILD ASICS ARE TYPICALLY SELECTED FOR LONG-TERM AVAILABILITY.”
To reduce ASIC obsolescence risk, the underlying silicon technologies used to build ASICs are typically selected for long-term availability. Medical device makers can typically secure 10–15 years of reliable component support – enough to span an entire product lifecycle without needing to revisit the design (Figure 2).
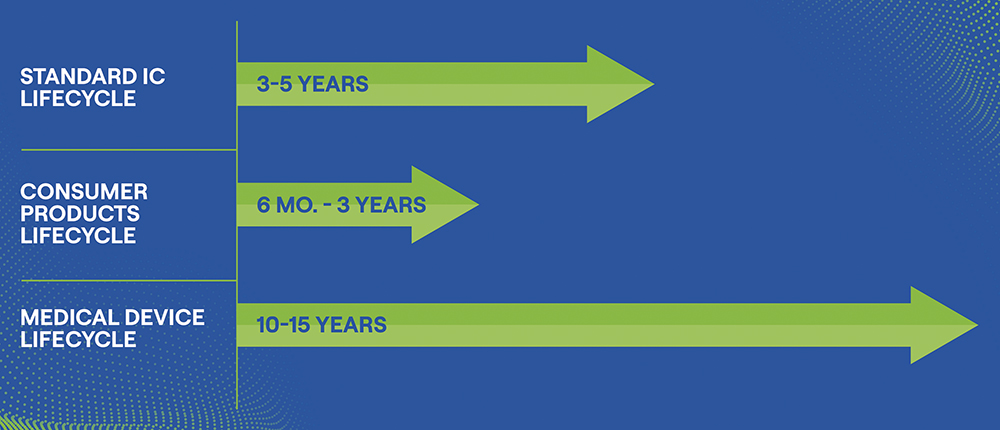
Figure 2: Component obsolescence lifecycle.
THE ASIC DESIGN PROCESS VERSUS STANDARD COMPONENTS
Designing an ASIC involves a different approach than traditional printed circuit board-level integration. Instead of selecting discrete ICs to perform specific functions, engineers define the desired behaviour and build those functions into a single integrated circuit. This enables:
- Miniaturisation: Consolidating multiple components into one ASIC dramatically reduces board size, which is critical for implantable devices.
- Power Optimisation: ASICs are tuned specifically for their use case, allowing for lower operating currents – enabling smaller batteries and longer battery life.
- Improved Reliability: Fewer interconnects and components can lead to fewer potential points of failure.
For some medical devices, ASICs are not just advantageous, they are essential. Applications requiring ultra-low power consumption, extreme miniaturisation or highly unique requirements cannot typically be achieved with standard components alone.
THE SUPPLY CHAIN BENEFITS OF ASICs
While ASIC development may require higher upfront investment, the long-term benefits are substantial. By avoiding mid-lifecycle redesigns, companies can save millions on redesign and re-validation costs, and prevent costly interruptions to product availability. In high-volume applications, ASICs can also offer unit cost savings by reducing the number of components, simplifying manufacturing processes and improving test yield.
REAL-WORLD EXAMPLES
These benefits are not just conceptual – they are already being realised in a range of advanced medical applications where ASICs are driving measurable improvements in performance, reliability and manufacturability – in addition to their obsolescence risk reduction. Examples include implantable neurostimulators, implantable cardiac pacemakers and defibrillators, implantable bio-sensors and implantable drug delivery devices. These typically include a wide range of analogue, digital and mixed signal circuits that are optimised to work in harmony to meet a very specific set of requirements. In most cases, these implantable devices would be impractical at best, and likely impossible to realise, without the use of ASIC-based circuit designs.
THE ROLE OF ASICs IN THE NEXT DECADE
Semiconductor technology innovation will continue and likely accelerate throughout the next decade. This means that the obsolescence risk of standard semiconductor components will continue and become even more prevalent.
Going forward, the medical ASIC industry will gradually evolve towards denser, smaller-geometry semiconductor technologies. This evolution is likely to enable increased integration of microcontroller units, self-checking circuits, redundancy mechanisms and built-in test functions into ASIC designs that, historically, have been analogue-centric. This hybridisation will bring more programmability, diagnostics and data handling capabilities to ultra-low power applications, enabling more intelligent devices that can adapt and self-correct in real time.
EMERGING TECHNOLOGIES TO SUPPORT MINIATURISATION
As the semiconductor industry progresses to smaller-geometry technologies, the standard operating voltages used in ASICs will be reduced and result in further reduction in power consumption, which will support the design of even less invasive devices that can improve patient comfort and reduce implant risk.
Along with semiconductor technologies, ASIC packaging technologies are also evolving rapidly. Wafer-level chip-scale packaging and 3D stacking can allow designers to push the boundaries of miniaturisation even further.
“WITH THE CONTINUOUS DEMAND FOR SMALLER, MORE CAPABLE AND LONGERLASTING DEVICES, ASICS WILL PLAY A PIVOTAL
ROLE IN REDUCING POWER CONSUMPTION WHILE INCREASING FUNCTIONALITY AND PERFORMANCE – WITHOUT OMPROMISING
SAFETY OR RELIABILITY.”
These advancements are positioning ASICs as key enablers of the next generation of implantable medical devices. With the continuous demand for smaller, more capable and longer-lasting devices, ASICs will play a pivotal role in reducing power consumption while increasing functionality and performance – without compromising safety or reliability.
PREPARING FOR THE FUTURE: PROACTIVE STRATEGIES FOR MEDTECH COMPANIES
To effectively realise the potential of ASICs and ensure long-term success, medtech companies should adopt proactive strategies throughout the product development lifecycle. These strategies include:
- Designing with Foresight: To fully realise the benefits of ASICs, medical device developers should consider their feasibility from the earliest stages of concept development. ASICs are less effective in designs that were not built with custom integration in mind. Early planning enables smoother integration of all device functions and better alignment with regulatory documentation and validation protocols.
- Anticipating Industry Shifts: Proactively exploring ASICs also positions companies to stay ahead of major industry trends, including wireless power and communications standards, and battery innovations that enable smaller, more efficient devices.
- Building Strategic Partnerships: Engaging with ASIC design and manufacturing partners during initial feasibility assessments can help teams to evaluate trade-offs in size, performance and cost while ensuring that long-term supply and manufacturability are built into the product roadmap. This foresight also supports more efficient clinical and regulatory timelines by reducing the risk of design changes late in the process.
REFERENCE
- “State of the U.S. Semiconductor Industry 2024”. Research report, Semiconductor Industry Association, 2024.

