To Issue 177
Trappler E, Miller D, Thomas M, “From Hospital to Home Healthcare: Advances in Lyophilised Product Delivery”. ONdrugDelivery, Issue 177 (Sep/Oct 2025), pp 66–71.
Edward Trappler, Denise Miller and Michael Thomas discuss the challenges for lyophilised products and consider the role of dual-chamber devices in simplifying reconstitution, particularly for at-home healthcare products, and go on to look at how future advances in delivery devices are providing safe solutions for self-administration while improving patient adherence.
Lyophilised products have been available since the mid-1930s, with plasma being one of the earliest applications of freeze-drying.1 Since then, lyophilisation has become essential for preserving a wide variety of pharmaceutical products. These include anti-infectives and vaccines, encompassing chemical and biochemical entities that span small molecules, natural and plasma proteins, to recombinant biologicals. Figure 1 illustrates the range of products in common conventional presentations.
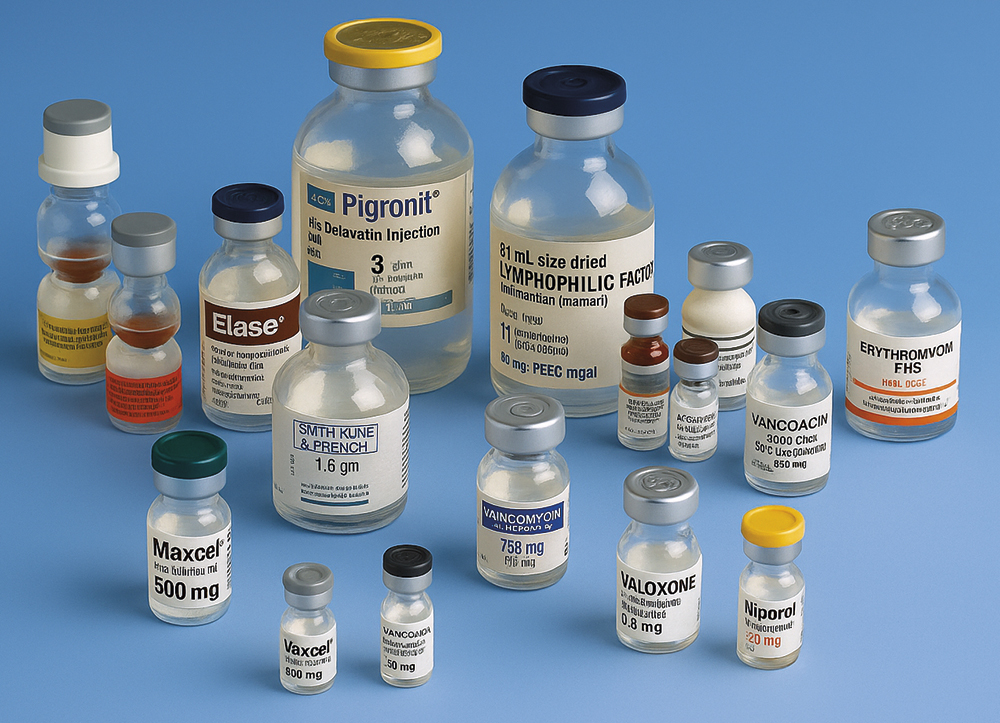
Figure 1: An array of presentations of parenteral products.
Preparing a lyophilised product for parenteral administration requires reconstituting the dried “cake” into a sterile liquid suitable for injection. This preparation step occurs in diverse settings – for example, in a hospital pharmacy, when preparing an anti-infective prior to surgery as a prophylactic treatment; in an institutional setting, such as for oncology therapy; in a clinic for vaccine administration; as part of a “crash trolley” (“crash cart”) in emergencies; in the field by a healthcare professional; or even by patients themselves in at-home healthcare.
QUALITY ATTRIBUTES
The standard requirements for any parenteral product include quality, purity and efficacy. Quality ensures that the product is suitable for its intended use. A level of purity entails physical, chemical/biochemical and microbiological attributes, and it is free from any contamination. Efficacy is demonstrated in clinical studies and confirmed that there is the required potency at batch release.
“LYOPHILISED PARENTERALS, HOWEVER, EMBODY A UNIQUE SET OF ATTRIBUTES IN ADDITION TO THOSE REQUIRED OF ANY INJECTABLE.”
Any parenteral product needs to meet the requirements as described in USP General Chapter 1, Injections.2 Lyophilised parenterals, however, embody a unique set of attributes in addition to those required of any injectable. Critical quality attributes that define success or failure in lyophilised presentations include residual moisture, full dissolution to form a homogenous composition and acceptable reconstitution time. A “pharmaceutically elegant” cake appearance is also desirable.
Lyophilised products are preserved by preventing degradation from hydrolytic reactions between the active drug substance and water. To achieve this, the residual water content must be reduced to sufficiently low levels during the lyophilisation process, and the potential for water ingress through the primary packaging during storage must be mitigated. For a conventional vial and stopper combination, the elastomeric closure must provide adequate barrier properties, and the glass vial and elastomer must achieve container closure integrity. This requirement places unique demands on cartridges and syringes, as the plunger separating the dried product from the diluent must not permit water permeation throughout the product’s shelf life.
Inspection adds an additional challenge for lyophilised products. When converted from a solution to a dried solid during the lyophilisation process, only the surfaces of the finished product cake can be observed, hindering inspection of the entire contents of the vial. Lyophilised products are therefore categorised as difficult-to-inspect. The 100% physical inspection of the dried product is to be supplemented with inspection of constituted samples of the lot.3
Once diluent is added, the dried product must dissolve completely to form a homogeneous solution with all required parenteral quality attributes. The method of reconstitution depends on the sensitivity of the active drug substance to stresses encountered during mixing. Small molecules generally dissolve readily, however, biologics such as peptides and proteins may be sensitive to shear, turbulence and at the air-liquid interface, which can lead to aggregation (Figure 2).4

Figure 2: Reconstituting a lyophilised product to form a homogenous solution and improper technique causing aggregation of a protein parenteral.
In conventional lyophilised preparations, such as those shown in Figure 1, reconstitution requires multiple components: the vial of lyophilised product, a container of liquid diluent, a syringe, two needles and alcohol wipes. Assembling these items is just the beginning of an 18-step process that starts with removing a protective cap on the diluent and ends with withdrawing the constituted solution for administration. For trained personnel, these steps can be completed in as little as 100 seconds; for someone unfamiliar, the process can take up to 12 minutes (unpublished data, Lyophilization Technology, Inc).
“THE DURATION OF DISSOLUTION IS INFLUENCED PRIMARILY BY THE PHYSICOCHEMICAL CHARACTERISTICS OF THE ACTIVE DRUG SUBSTANCE AND FORMULATION EXCIPIENTS, WITH PRODUCT VOLUME PLAYING ONLY A SECONDARY ROLE.”
Although reconstitution time is often defined as the interval required for dissolution after diluent addition, in practice it includes the entire procedure: preparation, mixing and withdrawal of the final solution. The duration of dissolution is influenced primarily by the physicochemical characteristics of the active drug substance and formulation excipients, with product volume playing only a secondary role.
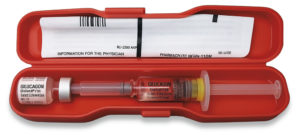
Figure 3: An “emergency kit” for treatment of an acute life-threating condition.
The clinical significance of reconstitution time varies by product. For Vancomycin Hydrochloride for Injection (Pfizer), an anti-infective available in a 10 g pharmacy bulk package, the time required is not critical. Reconstitution and further dilution occur in the hospital pharmacy before intravenous (IV) infusion, and delays pose little impact beyond inconvenience. Dantrolene, a component of an operating room crash trolley for malignant hyperthermia, must be prepared immediately. Up to 36 vials may be required for the patient, with two nurses often working together – one reconstituting while the other administrates the doses – to keep up with the patient’s needs. Here, reconstitution time can determine survival. At the extreme, glucagon injections for the treatment of hypoglycaemia require near instantaneous dissolution. During insulin shock, each second is precious, as delays can lead to dizziness, weakness, seizures and potentially coma. For this reason, glucagon is supplied in emergency kits where all necessary items are packaged together (Figure 3).
COMBINING DRUG AND DILUENT
To improve methods of reconstitution, Abbott Laboratories developed the ADD-Vantage system™ (now a Pfizer product), which introduced significant convenience for hospital pharmacies. This design allowed a lyophilised product vial to connect directly to an IV bag, with the IV solution itself serving as the diluent. The system streamlined workflow, eliminated multiple steps and enhanced sterility assurance (Figure 4). Abbott later introduced the ADD-Vantage ADDapter™ device, allowing conventional vials to connect to the specialised IV bag port. Despite its success, Pfizer has announced it is discontinuing the presentation after 2025.5
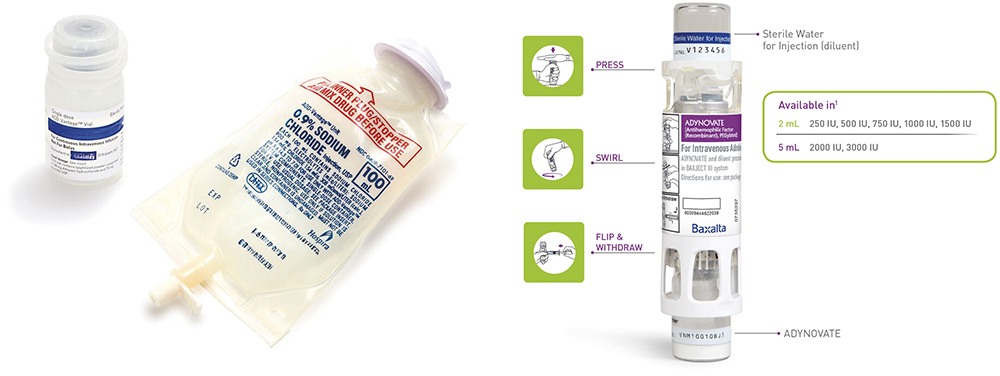
Figure 4: ADD-Vantage IV Mixture system and Baxject delivery of reconstitution protein for self-administration in home healthcare.
Further innovation came with dual-chamber devices designed to simplify reconstitution for home healthcare products, particularly those used to treat chronic conditions. These assemblies combine a vial of a lyophilised drug product and a vial of the diluent into a single device specifically configured for reconstitution. The Baxject unit (Takeda, Tokyo, Japan), for example, is used for administering antihaemophilia Factor VII (Figure 4).
Activation of the system begins by pressing on the diluent vial, which transfers the liquid into the product vial under reduced headspace pressure. This transfer is facilitated by a syringe attached to the side of the assembly via a Luer lock connection, which also serves later to withdraw the constituted solution for administration. Once the diluent has entered the bottom vial, the assembly is gently swirled to complete dissolution of the dried product to form a homogenous solution. The constituted solution can then be withdrawn into the syringe through the Luer lock connection and prepared for injection. The final preparation step involves attaching a needle to the syringe, making the dose ready for administration. Although great efforts are employed to make such delivery systems easy to use, patient compliance continues to be a significant consideration.6
Dual-chamber systems offer clear advantages: fewer steps, shorter preparation times, assurance of correct diluent volume, reduced contamination risk and lower needlestick potential. Reconstitution times were reported to range from 20 to 65 seconds, depending upon the age of the user.7 In addition, there is a greater likelihood that the correct amount of diluent will be added compared with traditional vial-to-vial transfers. Because dual-chamber configurations function as a more closed system, the potential for contamination is greatly reduced, sterility assurance is higher and needle handing is only required at the point of administration, thereby decreasing the risk of needlestick injuries.
“BUILDING ON THE SUCCESS OF DUAL-CHAMBER DEVICES, FURTHER DEVELOPMENT HAS LED TO SINGLE PRIMARY PACKAGING UNITS CONFIGURED SIMILARLY TO SYRINGES THAT INTEGRATE BOTH THE CONTAINER AND DELIVERY DEVICE INTO ONE SYSTEM.”
There has been an increased interest in products that can be self-administered in a home healthcare setting. As patients have grown accustomed to this convenience, demand has risen for prefilled syringe presentations for lyophilised products. Building on the success of dual-chamber devices, further development has led to single primary packaging units configured similarly to syringes that integrate both the container and delivery device into one system. These designs provide enhanced assurance of quality and purity while simultaneously improving patient compliance.
In these integrated systems, the dried product and diluent are contained within the same primary packaging, reconstituted directly inside the device and then delivered through the same unit. This approach minimises manipulations, reducing preparation just to steps essential for self-administration. By simplifying the process, the system decreases the risk of handling errors, limits opportunities for contamination and improves sterility assurance. One example is Abbvie Laboratories’ LUPRON DEPOT® (leuprolide) used for the management of endometriosis, including pain relief and reduction of endometriotic lesions.8 Supplied in a ready-to-administer format, it allows reconstitution and delivery in just a few simple steps, offering a patient-friendly alternative to traditional vial-and-diluent combinations.
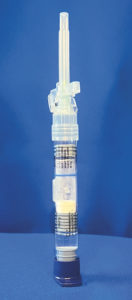
Figure 5: LUPRON DEPOT® has a ready-to-administer format.
Figure 5 shows the product in a ready-to-administer configuration that reduces the reconstitution process from the 18 steps described earlier to just three. The patient attaches the plunger rod, pushes the plunger past the bypass in the syringe barrel to allow the diluent to enter the chamber with the dried product, then gently inverts the syringe back and forth until reconstitution is complete. After these three simple steps, the product is ready to administer.
This type of presentation also reduces preparation time compared with dual-vial configurations. It ensures that the correct volume of diluent is delivered, eliminating uncertainty and further reducing user error. Because the process occurs entirely within a closed system, the potential for contamination is minimised, sterility assurance is maximised and needle handling is only required at the point of administration. This not only enhances safety but also decreases the risk of needlestick injuries.
DELIVERY DEVICES IN THE FUTURE
As new products for chronic conditions continue to be developed, the range of therapies suitable for home use has expanded. This reflects an era of unprecedented innovation in drug development. Many of these emerging therapies present delivery challenges, often requiring larger volumes, higher viscosities or novel formulations that exceed the capabilities of traditional technology.
To meet these demands, advances in delivery devices are providing solutions that enable safe self-administration while improving patient compliance. One such development is the introduction of systems that combine a reusable power unit with an interchangeable drug cassette, capable of accommodating both prefilled syringes and cartridges. These platforms offer greater flexibility in handling different drug formats, viscosities and volumes, supporting a wide variety of formulations and therapeutic regimens.
“BY REDUCING THE NUMBER OF MANUAL STEPS AND HANDLING REQUIREMENTS, THESE DEVICES MAKE COMPLEX THERAPIES MORE ACCESSIBLE FOR PATIENTS AND HELP ENSURE THAT TREATMENTS ARE DELIVERED SAFELY AND CONSISTENTLY IN THE HOME, ACHIEVING
In practice, such systems are designed for ease of use – the patient simply loads a cassette, removes the protective cap and presses the device against the injection site. By reducing the number of manual steps and handling requirements, these devices make complex therapies more accessible for patients and help ensure that treatments are delivered safely and consistently in the home, achieving improved therapeutic outcomes.
Connectivity is also another feature, benefitting both the patients and primary care providers. A radio frequency identification (RFID) tag on the product cassette can communicate the drug information to the device, which automatically adjusts delivery parameters, such as depth and injection duration, providing improved patient compliance. This simplifies the administration process for the patient and reduces the risk of errors.
Cellular connectivity can also be integrated into the delivery device, further improving patient compliance. Control of the power unit can be automated by the RFID-enabled disposable drug cassette, ensuring that the proper delivery parameters are used without the need for user input or creating opportunities for human error. Capabilities for control of such devices by healthcare providers offer unique capabilities for patients where dosing may be variable or determined by factors expected to change over time. The flexibility to deliver multiple volumes and use a variety of primary containers allows patients to use the same power unit with multiple drug cassettes. Without requiring patient input, there is no risk of the wrong dose being delivered and the user experience is identical, if not improved.
ON THE HORIZON
In the ever-changing landscape of parenteral drug delivery, new systems must evolve to accommodate an increasingly diverse range of product formulations and presentations. As therapeutic innovation accelerates, the growing complexity of treatments introduces new challenges and unmet needs for both patients and healthcare providers. Device technologies are advancing in parallel with the development of more capable and flexible autoinjectors that improve usability and adapt to novel product requirements.
Beyond supporting approved therapies, these innovations are also valuable in drug development. Next-generation delivery platforms play a crucial role in clinical research, enabling dynamic dosing strategies, accelerating evaluation, improving adherence and de-risking development through consistent administration. Connectivity features enhance these benefits by providing real-time data availability and integrity, allowing healthcare providers and developers to monitor usage, outcomes and compliance more effectively.9
Taken together, these advances are redefining what is possible for drug delivery in the home healthcare setting. The shift from multistep vial-and-diluent systems to integrated, connected platforms, including dual-chamber cartridges and syringes, is not only simplifying preparation but also expanding access to complex therapies. By reducing user burden, improving sterility assurance and enabling safe self-administration, these systems are making advanced treatments more practical. As delivery devices continue to evolve, patients and providers alike will benefit from safer, smarter and more adaptable solutions that support the future of pharmaceutical innovation.
REFERENCES
- Flosdorf E et al, “Procedure and apparatus for preservation in “lyophile” form of serum and other biological substances”. J Immunol, 1935, Vol 29, pp 389–425.
- “Injections and Implanted Drug Products (Parenterals) – Product Quality Tests”. US Pharmacopeia, Chapter 1, Mar 2016.
- “Visual Inspection of Injections”. US Pharmacopeia – National Formulary, Chapter 1790, 2021.
- Snell J et al, “Particle Formation and Aggregation of a Therapeutic Protein in Nanobubble Suspensions”. J Pharma Sci, 2016, Vol 105(10), pp 3057–3063.
- “Azithromycin for Injection, USP – ADD-VANTAGE® Dosage and Administration”. Pfizer, accessed Aug 2025.
- “Troubleshooting II your BAXJECT® Needle-less Transfer device”. Takeda, accessed Aug 2025.
- Ueda H et al, “Convenience Comparison Study of Reconstitution Devices for the Blood Coagulation Factor VIII Products Rurioctocog Alpha Pegol and Antihemophilic Factor (Recombinant”. Int J Gen Med, 2020, Vol 13, pp 1469–1476.
- “LUPRON DEPOT-Ped®”. Abbvie, May 2025.
- Masciopinto V, “SHL Medical’s Elexy™: Redefining Possibilities”. ONdrugDelivery, Issue 166 (Oct 2024), pp 78–82.

