To Issue 180
Citation: Cuevas Brun E, “Navigating the Regulatory Pathway for Next-Generation Nebulisers in Combination with Biologics.” ONdrugDelivery, Issue 180 (Nov 2025), pp 20–24.
Edgar Hernan Cuevas Brun discusses the role of nebulisers in delivering larger doses of fragile biologics, delving into the global regulatory landscape that must be understood in order to deliver these drugs on the market.
“DUE TO THE HIGH VALUE OF BIOLOGICS, EFFICIENT, OPTIMISED DELIVERY IS PRIORITISED ALONGSIDE THE NEED FOR CONSISTENT
AND RELIABLE DOSING, RESULTING IN A PREFERENCE FOR NEXT-GENERATION NEBULISERS WHEN DELIVERING BIOLOGIC MEDICATIONS.”
The development of new biologic formulations for delivery via inhalation has continued to advance in recent years with several novel therapeutics reaching clinical stages.1 When it comes to liquid formulations, identifying a suitable device to effectively deliver the API is essential. Nebulisers are often the preferred delivery system for therapies that require aerosolisation of larger doses.
One of the major challenges for the delivery of biologics using nebulisers is the stability and activity of the API post-nebulisation.2,3 Amongst the various established nebuliser technologies, mesh nebulisers are often considered to be the most suitable option for avoiding high shear forces and heat generation, which is critical as these factors may lead to aggregation, thermal denaturation or other undesirable effects. Due to the high value of biologics, efficient, optimised delivery is prioritised alongside the need for consistent and reliable dosing, resulting in a preference for next-generation nebulisers when delivering biologic medications.
The availability of next-generation nebulisers is still limited compared with more traditional devices. This can be attributed to several factors, including the complexity of development, a lack of clear guidelines for new features and reimbursement obstacles in some markets. The regulatory pathway plays an essential role in market access for next-generation nebulisers. Therefore, gaining a suitable understanding of these pathways can lead to a more effective development strategy for their use in combination with biologics.
BASIC APPROVAL PATHWAYS
The approval pathway for medical devices varies from one market to another. When considering two of the major global markets, the US and the EU, nebulisers are classified as Class II devices based on their intended used and risk level (moderate risk). Further subclassification is found in the EU, where they are divided into Class IIa and IIb.4
The regulatory pathway for general-purpose nebulisers can be summarised as follows:
- US Market, 510(k) Premarket Notification: This pathway focuses on substantial equivalence to a predicate device in terms of intended use, technology and safety.
- EU Market, Medical Device Regulation (MDR EU 2017/745): This regulation defines the requirements to obtain CE marking focusing on safety, performance and clinical benefit. The approval process involves a notified body and requires clinical evaluation.
In the drug-nebuliser combination space, the main difference is that the US FDA does not require the nebuliser to be 510(k) cleared. The device is approved as part of the combination product package, with the key institution being either the Center for Drug Evaluation and Research or the Center for Biologics Evaluation and Research.5 For the EU market, drug-nebuliser combination products that comply with MDR Article 117 can be submitted with the nebuliser as part of the drug marketing authorisation application.6
Clarifying basic regulatory requirements is the first step towards comprehending the challenges of next-generation nebuliser development in terms of market access.
UNDERSTANDING THE IMPLEMENTATION OF EXISTING GUIDELINES
Existing guidelines cover different aspects of the device to ensure that a nebuliser is safe and efficacious. In the US, the FDA grants approvals, whereas, in Europe, marketing authorisations are granted by the EMA. When it comes to performance assessment, there are three major guidelines: ISO 27427:2023 – Nebulising Systems and Components, US Pharmacopeia (USP) Chapter <1601> and European Pharmacopoeia (EP) Chapter 2.9.44 (Table 1).7–9 ISO 27427:2023 is an international standard for nebulising systems and components, and it serves as the basis for testing guidelines. It is commonly used as the standard for nebuliser manufacturers of general-purpose devices.
| ISO 27427:2023 | USP <1601> | EP Chapter 2.9.44 | |
| Region | International (especially EU via MDR harmonisation) | US | Most of Europe |
| Type | Device performance standard | Pharmacopoeial chapter | Pharmacopoeial chapter |
| Scope | General-purpose nebulisers (device-centric) |
Nebulised drug products (drug-centric) |
Nebulised drug products (drug-centric) |
| Applies to | Medical device manufacturers | Drug and device manufacturers | Drug and device manufacturers |
| Test Substance | Uses standard solutions (e.g. 0.1% albuterol in 0.9% NaCl) |
Uses actual drug product | Uses actual drug product |
| Main Use | Device benchmarking, CE marking (EU), support 510(k) (US) |
Drug registration – NDA/ANDA (US) |
Marketing authorisation (EU and Canada) |
Table 1: Main guidelines for the assessment of nebulisers’ aerosol performance.
The test substance is often saline with albuterol, which is used for obtaining CE marking and, in some cases, to support 510(k) packages. The USP and EP guidelines apply to device and drug manufacturers and are drug specific. In the case of biologics, testing aerosol performance is not sufficient, since stability and activity post-nebulisation need to be confirmed, resulting in additional test requirements.
Other aspects that need to be satisfied according to the guidelines are also dependent on the territory, with the MDR requiring a risk management assessment as per ISO 14971:2019, biological safety as per ISO 10993 and ISO 18562, software validation as per IEC 62304 and usability as per IEC 62366, amongst other requirements. Similarly, a 510(k) premarket notification submission package comes with requirements for analogous aspects, including quality system upgrades as per QMSR/ISO 13485:2016.
Due to the complexity of the guidelines and documentation landscape, it is indispensable to have an experienced group of experts to generate comprehensive packages for submission. The degree of complexity increases significantly when developing next-generation nebulisers, and even more so for drug-nebuliser combination products.
APPROVAL CHALLENGES FOR NEXT-GENERATION NEBULISERS
Next-generation nebulisers are equipped with features that can support the effective delivery of biologics; however, only a few of these devices have been approved in major markets. Moreover, the regulatory pathway for a general-purpose device and a customised nebuliser for a specific drug-device combination vary significantly. Therefore, mapping a clear regulatory pathway from the start of development is a crucial step for a successful market launch and is a core factor for nebuliser developers and manufacturers.
When it comes to the approval of innovative products, challenges related to generating documentation packages for submission may arise not only from the complexity added by the new technologies but also from the lack of clear guidelines, or even from commercial obstacles such as reimbursement issues.
“THE FIRST CONNECTED NEBULISER WAS CLEARED IN 2020, AND THE ADHERESP NEBULISER IS THE FIRST BREATH-ACTUATED MESH NEBULISER TO BE CLEARED WITH THIS FUNCTION AS PART OF ITS SUBMITTED PACKAGE.”

Figure 1: AdheResp® Smart Breath-Actuated Nebuliser.
A successful example of the submission of an innovative nebuliser, which had to overcome the aforementioned obstacles, is the approval of the AdheResp Smart Breath-Actuated Mesh Nebuliser, designed and manufactured by HCmed Innovations (Figure 1). This connected, breath-actuated device was cleared in June 2025 under the traditional 510(k) pathway. The submission strategy was initiated by defining suitable predicate and reference devices in order to cover the key aspects of the package (Figure 2). AdheResp was specifically developed to deliver high value drugs with three innovative features that can significantly enhance the delivery of biologics:
- Breath Actuation: This mechanism allows aerosol delivery only during a fraction of the inhalation phase. The breath-actuated mode in AdheResp is adaptive, which means that it can predict the length of the next inhalation by computing the average of previous ones. Moreover, the amount of aerosol that is delivered during each inhalation can be adjusted to allow for chase air to pass through the device and enhance delivery to the lungs. Only a few breath-actuated nebulisers have received 510(k) clearance in the past two decades. Amongst them, the I-Neb AAD system (Philips, Amsterdam, Netherlands) was the first breath-actuated mesh nebuliser and then, most recently, the AdheResp nebuliser was also cleared with this function.7,8
- Connectivity: Aiming to support patient adherence monitoring, this feature allows for data transmission from the nebuliser to a mobile device. The first connected nebuliser was cleared in 2020, and the AdheResp nebuliser is the first breath actuated mesh nebuliser to be cleared with this function as part of its submitted package.9
- Activation: Designed for drug-specific nebulisers, the activation feature binds the use of the nebuliser with the specific drug in order to ensure safety. RFID technology is commonly used for this function, and only a few devices have incorporated it to date.
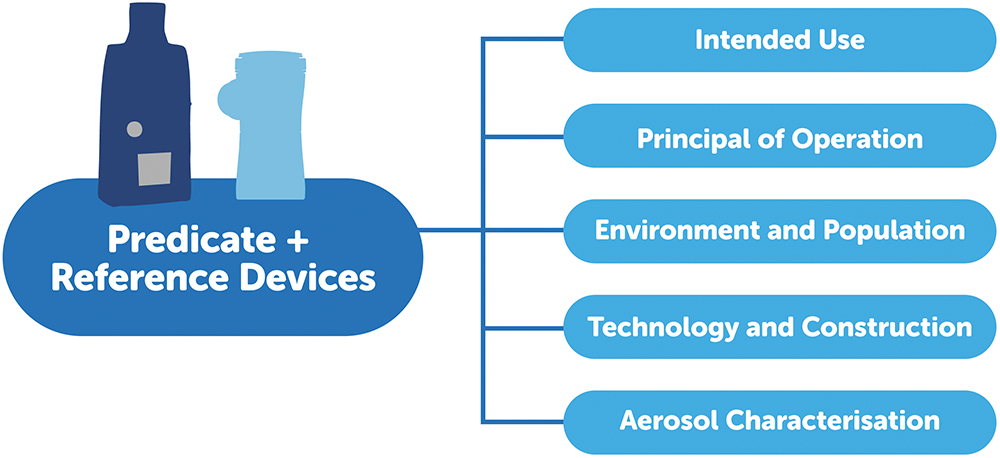
Figure 2: Substantial equivalence aspects for predicate and reference devices required in 510(k) premarket notification submission package.
The features listed above bring substantial value to AdheResp, however, the challenges involved in incorporating them extend from the lack of specific testing and apparatus guidelines to the limited number of breath-actuated nebuliser (BAN) predicates and the additional considerations required by strict software and cybersecurity regulations (Figure 3). The clearance of the AdheResp nebuliser is, without a doubt, a positive case, but the points mentioned here may be the main factors discouraging adoption of these technologies, thus reducing their market availability.
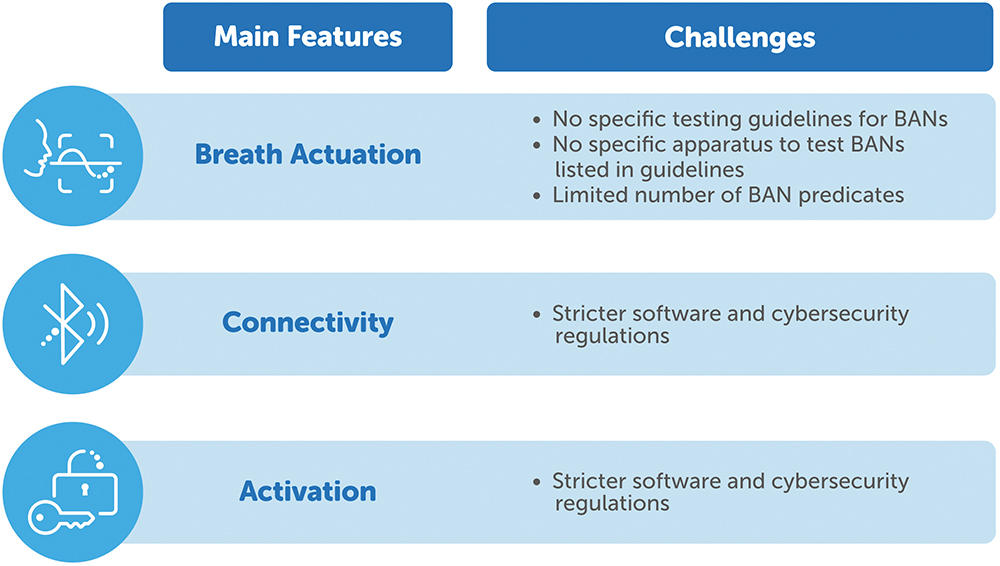
Figure 3: Challenges for the incorporation of new technologies in nebulisers.
The development of inhaled biologics in liquid form can be directly influenced by these factors. This has generated the demand for real solutions that can guarantee the use of approved nebulisers during early clinical phases with customisation capabilities for further optimisation of the commercial product.
FINDING THE RIGHT SUPPORT FOR DEVELOPMENT
CDMOs like HCmed Innovations provide support for drug-nebuliser combination products. HCmed’s strategy focuses on having its standard devices for each platform primed for clinical use. This approach facilitates readiness for clinical trials, including ensuring that a sufficient volume of devices are available.
HCmed’s platforms can be customised based on performance and usability requirements for drug-nebuliser combination products. In most cases, tailoring nebulisers for drug-specific delivery can trigger the initiation of a new design along with the respective design validation and verification processes of the customised device. One of the main benefits of counting on nebuliser platforms is that some of the documents from approved standard devices can be reused for customised versions, thus de-risking development and shortening overall development timelines.
“AN IMPORTANT PART OF THIS IS THE REGULATORY EXPERTISE THAT ELABORATES THE STRATEGY FOR SUBMISSION AND COMPILES THE DOCUMENTATION TO DEMONSTRATE THE SAFETY AND EFFICIENCY OF THE DELIVERY SYSTEM.”
For large pharmaceutical companies or small- and medium-size biotech companies, having the right support for nebuliser development processes is crucial to these projects. An important part of this is the regulatory expertise that elaborates the strategy for submission and compiles the documentation to demonstrate the safety and efficiency of the delivery system.
Considering the high value of biologic formulations, finding the right support from early development is essential to arrange an appropriate strategy and ensure compliance from the nebuliser perspective of the combination product. This is certainly more critical when working with next-generation nebulisers, where an experienced partner in the field can make navigation through the complex regulatory pathway to market access significantly easier.
REFERENCES
- Shaibie NA et al, “Inhaled biologics for respiratory diseases: clinical potential and emerging technologies”. Drug Deliv Transl Res, 2025, Vol 15(11), pp 4098–4114.
- Montefusco-Pereira CV, “Steps toward nebulization in-use studies to understand the stability of new biological entities”. Drug Discov Today, 2023, Vol 28(2), art 103461.
- Ibrahim M et al, “Protein Aggregates in Inhaled Biologics: Challenges and Considerations”. J Pharm Sci, 2023, Vol 112(5), pp 1341–1344.
- “MDCG 2021-24 – Guidance on classification of medical devices”. European Commission, Oct 2021.
- James Heinl, “Chapter 78 – Combination products” in “Handbook for Designing and Conducting Clinical and Translational Research” (Eltorai AEM, Hartnett DA, Bakal JA, Tereshchenko LG, eds). Academic Press, Translational Pulmonology, 2025, pp 419–424.
- “An Industry Perspective on Article 117 of the EU Medical Devices Regulation and the Impact on how Medicines are Assessed”. EFPIA, Reflection Paper, Jul 2018.
- “I-neb Insight AAD System Special 510(k) Premarket Notification Tab 3”. PDF, US FDA, Dec 2005.
- “AdheResp Smart Breath-actuated Mesh Nebulizer”. Letter, US FDA, Jun 2025.
- “AireHealthTM Nebulizer”. Letter, US FDA, Dec 2020.

