To Issue 181
Citation: Bovier E, “Mapping Innovation in mRNA Delivery Through Patent Trends”. ONdrugDelivery, Issue 181 (Dec 2025), pp 34–39.
Dr Elodie Bovier considers the evolution of messenger RNA therapeutics in biotechnology, examining patent trends and exploring how they have triggered litigation in the field, demonstrating the growth and maturity of this sector.
Messenger RNA (mRNA) therapeutics have evolved from a research concept to a central pillar of modern biotechnology, redefining the landscape of gene and cell therapies. One critical factor lies at the core of this transformation: the delivery system that determines not only therapeutic efficacy but also the feasibility of scaling these approaches across diverse indications.
THE CENTRAL ROLE OF DELIVERY IN mRNA THERAPEUTICS
Mechanistic Basis of mRNA Delivery
After administration, mRNA must survive in circulation, reach the correct tissue and enter target cells – impossible steps without a delivery system. Naked mRNA is rapidly degraded by serum nucleases and cleared by the liver and spleen. Effective carriers therefore protect the molecule, promote cellular uptake and enable endosomal escape – the crucial step that releases mRNA into the cytoplasm for translation. Each stage – protection, biodistribution and cellular entry – depends on the delivery system, making delivery design the true determinant of therapeutic success.1–4
LNPs: Established But Evolving
Among non-viral systems, lipid nanoparticles (LNPs) are the most clinically validated vehicles. They combine ionisable lipids, phospholipids, cholesterol and polyethylene glycol (PEG) lipids to condense and protect RNA in circulation. Ionisable lipids bind mRNA at a low pH and trigger release inside endosomes, while helper phospholipids stabilise membranes. Cholesterol adds rigidity, and PEG-lipids reduce aggregation and immune recognition – although excessive PEGylation can hinder uptake if desorption is delayed.2,4–6
Despite the success of LNPs, challenges remain – hepatic tropism, immunogenicity and limitations on repeated dosing. These constraints drive innovation in lipid chemistry, such as new ionisable scaffolds, biodegradable PEG substitutes and ligand–lipid conjugates for tissue targeting.
Innovation also extends beyond lipids to polymeric carriers and biological vesicles. As many advances precede clinical validation, patent activity functions as the earliest and most quantifiable signal of progress. Monitoring these dynamics provides a direct measure of how rapidly mRNA delivery is evolving.
PATENT ACTIVITY AS AN INNOVATION BAROMETER
Patent Trends in mRNA Delivery
Intellectual property (IP) analysis provides a powerful lens through which to trace the technological evolution of mRNA therapeutics. The data presented in this article focus on a timeline from Q1 2024 to Q3 2025, reflecting the most recent dynamics within the delivery segment.
“ACROSS ALL TECHNOLOGICAL SEGMENTS OF mRNA THERAPEUTICS, DELIVERY REMAINS ONE OF THE MOST ACTIVELY PATENTED AND STRATEGICALLY PROTECTED AREAS.”
During this period, about 1,450 mRNA-related patent families were published worldwide, of which nearly half (~49%) addressed delivery. The quarterly share ranged from 42% to 59%, peaking in Q2 2024 (59%) and Q2 2025 (54%). Even in quarters with slightly lower total publication volumes (e.g. Q3 2024 and Q4 2024), delivery maintained a consistent presence of above 45% of all filings. This persistence highlights that, across all technological segments of mRNA therapeutics (design, manufacturing, storage or application), delivery remains one of the most actively patented and strategically protected areas (Figure 1).
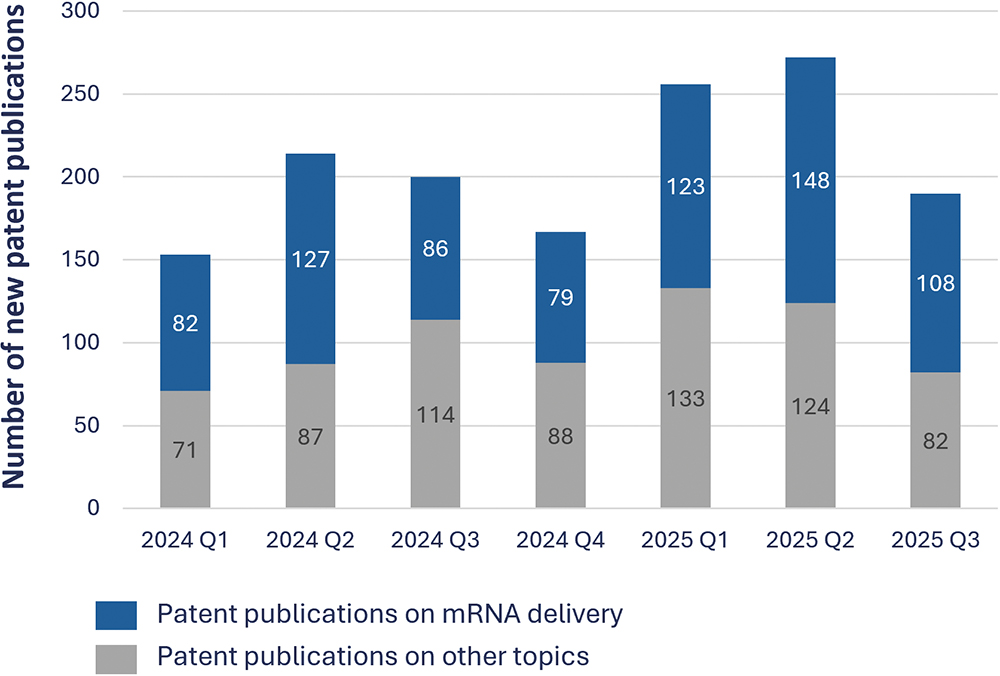
Figure 1: Quarterly proportion of delivery-related patent families, Q1 2024–Q3 2025.
This trend is further reflected within the profiles of the leading assignees in this field (Figure 2). The University of Pennsylvania (PA, US) leads delivery-related patenting, with 20 new publications in 2025 compared with nine the previous year, followed by BioNTech (Mainz, Germany), with a similar trajectory (17 versus eight new delivery patents year-on-year). In contrast, Moderna (Cambridge, MA, US) and Abogen (Suzhou, China), which dominated the 2024 landscape, have not yet appeared in 2025, likely due to pending disclosures.
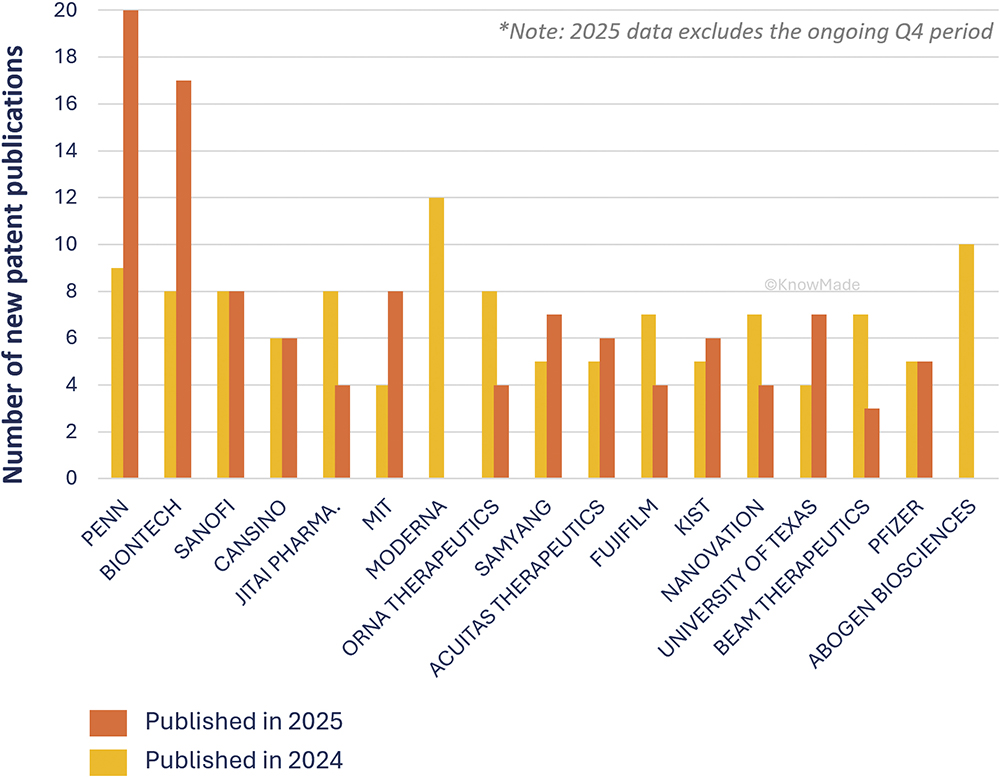
Figure 2: New patent publications for delivery by main assignees in 2024 and 2025*.
Other prominent players – including Sanofi, CanSino (Tianjin, China) and Pfizer – maintained stable levels of new patenting activity, while companies such as Jitai Pharmaceutics (Shaanxi, China), Orna Therapeutics (Watertown, MA, US), Fujifilm (Tokyo, Japan), Nanovation (Vancouver, BC, Canada) and Beam Therapeutics (Cambridge, MA, US) showed a slight decline. Conversely, a new wave of academic and research organisations, MIT (Cambridge, MA, US), Samyang Biopharmaceuticals (Seoul, South Korea), Acuitas Therapeutics (Vancouver, BC, Canada), KIST (Seoul, South Korea) and the University of Texas (Austin, TX, US), demonstrated higher activity in 2025 compared with 2024, underscoring academia’s growing role in advancing delivery platforms and broadening the innovation base of the field.
These shifts illustrate how patent dynamics act as an innovation radar, capturing both the speed and direction of technological changes. Data confirm that delivery is not only a scientific challenge but also a strategic axis defining competitiveness in the mRNA sector (Figure 2).
“A NEW GENERATION OF COMPANIES IS REIMAGINING RNA TRANSPORT TO OVERCOME IMMUNOGENICITY, TISSUE TARGETING AND SCALABILITY BARRIERS.”
BEYOND LNPs: INNOVATION FROM NEWCOMERS
While LNPs remain the cornerstone of mRNA therapeutics, the field is rapidly diversifying. A new generation of companies is reimagining RNA transport to overcome immunogenicity, tissue-targeting and scalability barriers. Their work demonstrates how delivery innovation has evolved from a domain dominated by major pharmaceutical players into a multidisciplinary frontier, with academic institutions and emerging companies playing an increasingly decisive role.
Among them, three companies illustrate how innovation in the post-LNP era is being driven by novel concepts that expand the possibilities of mRNA transport beyond conventional lipid-based architectures: Parcel Bio (San Francisco, CA, US), AGS Therapeutics (Paris, France) and CILA Therapeutics (Boston, MA, US).
Case Study 1: Parcel Bio – Molecularly Encoded Delivery
Parcel Bio (founded 2023) has introduced STAmP™ (Structured Trans-Assembly of mRNA Precursors), a nanoparticle-free delivery in which oligo-tiled, ligand-targeted mRNA forms stable molecular complexes without lipids. Patent WO 2025/166052 reports strong serum stability, protection from nuclease degradation and low innate-immune activation, while maintaining robust protein expression comparable with lipid-based formulations. By removing the lipid component, STAmP aims to support repeat dosing and broaden biodistribution to muscle and central nervous system targets, pointing to a shift towards molecularly encoded delivery, where the RNA architecture contributes to both stability and targeting (Figure 3a).
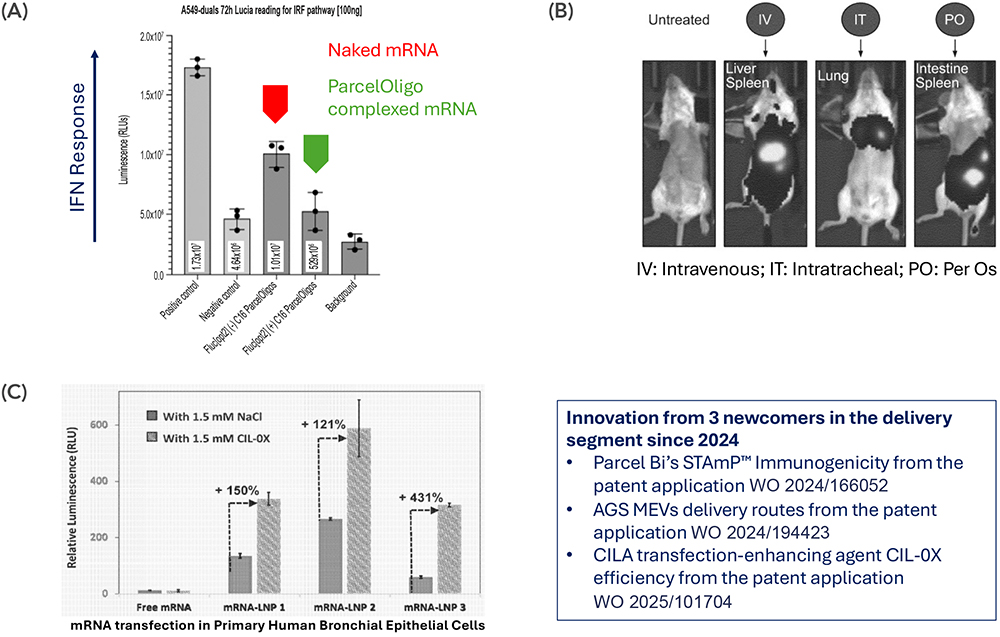
Figure 3: Representative newcomer delivery platforms disclosed in recent patents: (A) Parcel Bio STAmP™, (B) AGS Therapeutics MEVs, (C) CILA Therapeutics aerosol lipids.
Case Study 2: AGS Therapeutics – Microalgae Vesicles
In Europe, AGS Therapeutics (founded 2020) has developed a biologically inspired alternative using microalgae extracellular vesicles (MEVs) derived from Chlorella vulgaris. These vesicles are inherently non-immunogenic, biodegradable and scalable, capable of transporting RNA, DNA and proteins. Their natural origin and sustainable production process make them an attractive and eco-friendly alternative to synthetic nanocarriers. Patent WO 2024/194423 demonstrates that MEVs can cross mucosal and blood-brain barriers to deliver RNA and protein cargos in vivo (Figure 3b). Administered via oral, nasal or ocular routes, they achieved their functional objectives. Reported outcomes include antigen-specific immune responses and cytokine modulation without interferon activation or detectable toxicity, supporting repeatable administration.
Case Study 3: CILA Therapeutics – Pulmonary Delivery
Another emerging company, CILA Therapeutics (founded 2018) focuses on the pulmonary route – one of the most challenging targets for mRNA drug delivery. Its international patent application (WO 2025/101704) details a lipid-based aerosol formulation optimised for nebulisation, combining cationic and helper lipids to preserve RNA integrity and promote expression in airway cells. In addition, co-formulation with thiol compounds, such as MESNA or CIL-OX, improves uptake under oxidative conditions while maintaining cell viability, demonstrating that aerosolised lipid systems can achieve efficient, repeatable RNA delivery directly to the lung (Figure 3c). This approach is particularly relevant for diseases such as cystic fibrosis and primary ciliary dyskinesia.
Summary
Together, these newcomers illustrate a clear post-LNP diversification from LNP optimisation and alternatives such as molecular self-assembly, to biological vesicles and aerosol delivery. Such transitions, visible first in patent data, redefine the boundaries of mRNA pharmacology and suggest new paths for systemic and local delivery.
STRATEGIC LITIGATION AND THE CONSOLIDATION OF mRNA DELIVERY TECHNOLOGIES
Overview of Major Legal Disputes
The growing commercial value of LNP and delivery patents has triggered extensive litigation, reflecting the field’s maturity. Between 2020 and 2025, six major disputes shaped the LNP IP landscape, ranging from inventorship claims to infringement and trade-secret actions, as illustrated in Table 1.
These cases underline how ownership of delivery IP has become a decisive competitive determinant. Cross-licensing outcomes define royalty baselines, while ongoing suits will likely establish precedents for global mRNA patent enforcement.
| Year | Plaintiff | Defendant | Delivery Tech Focus |
| 2020–2025 (appeal) | Moderna | Pfizer/BioNTech | Partly delivery (5′ UTR & LNP claim overlap) |
| Cause of Action: Infringement Jurisdiction/Case: UK Court of Appeal Subject/Patent Family: mRNA construct & delivery patents EP3 719 565 B1 et al. Status*: Pfizer/BioNTech lost appeal Aug 2025 |
|||
| 2021–2025 | Arbutus Biopharma / Genevant Sciences | Moderna | Ionisable lipids & LNP formulation composition & manufacture |
| Cause of Action: Infringement Jurisdiction/Case: US District Court, Case 1:22-cv-00252 + Global fillings Subject/Patent Family: LNP delivery patents (Arbutus/Genevant portfolio) Status*: Active; trial set for Sep 2025 |
|||
| 2022–2025 | Alnylam | Moderna | Cationic/ionisable lipids for mRNA delivery |
| Alnylam | Pfizer | ||
| Cause of Action: Infringement Jurisdiction/Case: US District Court, Case. 1:23-cv-00580 (Alnylam v. Moderna) & 1:23-cv-00578 (Alnylam v. Pfizer) Subject/Patent Family: Cationic-lipid patents (Alnylam) used in mRNA vaccine LNPs Status*: Non-infringement in favour of Moderna (currently on appeal) & still pending for the other case |
|||
| 2022–2025 | CureVac/GSK | BioNTech & Pfizer | LNPs & ionisable lipid formulations |
| Cause of Action: Infringement & EPO Opposition Jurisdiction/Case: US District Court, Case 2:23-cv-00222 Subject/Patent Family: CureVac’s mRNA + delivery patents (‘493 Family & others) Status*: Settled (Aug 2025) – Pfizer/BioNTech paid $740 M + royalties; granted non-exclusive US licence, BioNTech’s deal to buy CureVac |
|||
| 2023–2024 | Acuitas Therapeutics | CureVac SE | LNPs – cationic lipid ALC-0315 for mRNA delivery |
| Cause of Action: Inventorship Jurisdiction/Case: US District Court, Case 3:23-cv-00764 Subject/Patent Family: US Patents 11,241,493 et al. (‘493 Family) Status*: Settled (Apr 25, 2024); inventorship dispute resolved, CureVac withdrew three patents; Acuitas licensed use rights |
|||
| 2024 | GSK | Moderna | LNP & self-amplifying mRNA delivery systems |
| Cause of Action: Infringement Jurisdiction/Case: US District Court, case 1:24-cv-01136 and 1:24-cv-01135 Subject/Patent Family: GSK mRNA/LNP platform patents (Moderna’s Spikevax & mRESVIA) Status*: Early stage |
|||
| 2025 | Arcturus Therapeutics | AbbVie (Capstan Therapeutics) | LNP formulation know-how for mRNA delivery |
| Cause of Action: Trade-secret suit Jurisdiction/Case: US District Court, case 3:25-cv-02494 Subject/Patent Family: Alleged misappropriation of LNP delivery tech Status*: Early stage |
|||
*As of October 2025.
Table 1: Summary of the six main litigations in the mRNA delivery field.
From Conflict to Collaboration
The settlements in Acuitas versus CureVac (Tübingen, Germany) and CureVac/GSK versus BioNTech and Pfizer illustrate a shift from defensive litigation to cross-licensing aimed at stabilising access to LNP technology. The Acuitas–CureVac dispute revolved around the patent family, covering mRNA–LNP formulations, incorporating the cationic lipid ALC-0315, used in Comirnaty®. Acuitas claimed CureVac excluded its scientists from inventorship; the case was settled in April 2024, with CureVac withdrawing disputed patents and granting Acuitas licences. In parallel, CureVac (with GSK) and BioNTech/Pfizer contested patents relating to mRNA sequence optimisation and LNP delivery. BioNTech and Pfizer sought declaratory judgements of non-infringement, while CureVac countered with infringement claims. The proceedings concluded in August 2025 through a global settlement of roughly US$740 million (£568 million) plus royalties, granting BioNTech and Pfizer non-exclusive US licences. Together, these cases exemplify how major players now employ IP negotiation to secure technological and market stability.
The Arbutus/Genevant Versus Moderna Precedent
In contrast, the ongoing Arbutus (Warminster, PA, US) and Genevant (Vancouver, Canada) versus Moderna proceedings, spanning more than 30 jurisdictions, represent the consolidation of foundational LNP patents as globally strategic assets and one of the most extensive global disputes to date concerning the IP on LNPs. Indeed, this case was filed initially in the US in 2021 and subsequently in five international lawsuits (in Canada, Japan and Switzerland, and two filed in the Unified Patent Court). Beyond its commercial scale, the case can be viewed as a potential precedent-setter for the valuation and licensing of core LNP technologies, as its outcome may establish the royalty structures and ownership boundaries that will govern future mRNA delivery partnerships across the industry.
“PATENT ACTIVITY QUANTIFIES INNOVATION MOMENTUM, IDENTIFIES NEW ENTRANTS AND CAPTURES THE DIVERSIFICATION OF MATERIALS LONG BEFORE CLINICAL VALIDATION.”
CONCLUSION: THE MATURATION OF DELIVERY AS A COMPETITIVE AXIS
The analysis of patent dynamics and litigation trends offers a unique vantage point on how mRNA delivery is evolving. Patent activity quantifies innovation momentum, identifies new entrants and captures the diversification of materials long before clinical validation. Litigation patterns, in turn, reveal where technology ownership and value are consolidating. For scientists, these developments expand the possibilities of nucleic-acid pharmacology; for analysts, they map the competition of tomorrow’s mRNA therapeutics market.
Delivery remains the decisive enabler of mRNA therapeutics — and the pulse of that innovation is written first in its patents.
REFERENCES
- Hamilton AG, Swingle KL, Mitchell MJ, “Biotechnology: Overcoming biological barriers to nucleic acid delivery using lipid nanoparticles”. PLoS Biol, 2023, Vol 21(4), art e3002105.
- Monique CP et al, “Design of lipid-based nanoparticles for delivery of therapeutic nucleic acids”. Drug Discov Today, 2023, Vol 28(3), art 103505.
- Moon S, Lee JB, “Lipid nanocarriers for RNA and DNA therapeutics: Next-generation delivery strategies in precision medicine”. J Ind Eng Chem, 2025, available online.
- Xu S et al, “Lipid nanoparticles: Composition, formulation, and application”. Mol Ther Methods Clin Dev, 2025, Vol 33(2), art 101463.
- Chowdhury CR, Hoover EC, Day ES, “Membrane-modified lipid nanoparticles for RNA delivery”. Mol Ther Methods Clin Dev, 2025, Vol 33(3), art 101505.
- Zhang T et al, “Optimized lipid nanoparticles (LNPs) for organ-selective nucleic acids delivery in vivo”. iScience, 2024, Vol 27(6), art 109804.

