To Issue 182
Citation: Moersch W, “Testing Autoinjectors According to ISO 11608-5 to Increase Reliability and Boost Efficiency”. ONdrugDelivery, Issue 182 (Jan 2026), pp 85–88.
Visit Zwick Roell at Pharmapack Paris! – Stand 4F124
Wolfgang Moersch discusses the role of automation in testing injection devices – including autoinjectors, pen injectors and on-body injectors – and how advanced testing software and robotics solutions can provide more accurate and consistent testing results.
Autoinjectors and advanced injection systems play a central role in the modern drug delivery landscape, particularly for biologics and self-administration therapies. As these combination products become more complex – handling higher viscosities, larger volumes and increasingly diverse patient needs – the demands on testing strategies have grown significantly. Robust, reproducible and traceable testing is no longer limited to late-stage quality control; it is now essential across the entire product lifecycle, from early development through to commercialisation.
“BRINGING AN AUTOINJECTOR TO MARKET REQUIRES ALIGNMENT BETWEEN DEVICE DESIGN, DRUG FORMULATION, REGULATORY EXPECTATIONS AND MANUFACTURING REALITIES.”
TESTING CHALLENGES IN A GLOBAL REGULATORY ENVIRONMENT
Bringing an autoinjector to market requires alignment between device design, drug formulation, regulatory expectations and manufacturing realities. Pharmaceutical companies and CDMOs must manage parallel development paths – design verification on the one hand and process validation and scale-up on the other. At each milestone, from design freeze and submission through to validation and routine production, testing data must be verifiable, comparable and fully documented.
The correct injection technique and proper dose of medicine are vital to achieve therapeutic success. Therefore, pharmaceutical manufacturers strive to achieve a high level of automation in autoinjector technology, where the patient simply removes the safety cap, positions the injector and injects the drug by pressing a button – the subsequent injection process is completely automated. This means that all relevant injector functions must be checked before production batches are released to the market. This testing is performed according to ISO 11608-5.
KEY PARAMETERS IN AUTOINJECTOR TESTING
A modern autoinjector test sequence typically combines multiple measurements within a single automated workflow (Figure 1). These include:
- Cap removal force, measured either upwards or downwards, depending on the intended patient handling defined in the instructions for use
- Activation force and travel, ensuring reliable device triggering
- Injection depth, confirming that the needle penetrates only within the intended range
- Injection time and flow behaviour, including the precise definition of “end of injection”
- Delivered volume, measured gravimetrically under controlled conditions
- Needle safety device activation and strength, where applicable.

Figure 1: Example test setup according to ISO 11608-5. The test sequence combines multiple measurements within a single test.
“INTEGRATING THESE MEASUREMENTS INTO A SINGLE TEST RUN ON ONE SPECIMEN IMPROVES DATA INTEGRITY AND ENABLES DIRECT CORRELATION OF RESULTS.”
Integrating these measurements into a single test run on one specimen improves data integrity and enables direct correlation of results. Automated systems further reduce operator influence – an important factor for comparability between R&D, quality control and external partners.
CAMERA-BASED MEASUREMENT AND TRACEABILITY
One notable development in autoinjector testing is the use of camera-based measurement systems. These allow non-contact detection of critical events, such as start and end of injection, needle exposure and liquid stream behaviour. High-speed, time-synchronised video capture improves accuracy, particularly for the very short injection times typical for emergency injectors.
Camera systems also support flexible definitions of “end of injection”, accommodating different regulatory or internal interpretations, such as last continuous stream, last drop or time-based extensions. Importantly, the ability to calibrate such systems ensures long-term measurement confidence and regulatory acceptance.
Traceability is further enhanced by automatic documentation of environmental conditions (temperature and humidity), test configuration, sensor status and visual records of the injection process. This level of documentation is increasingly expected during audits and inspections.
PREVENTING MEASUREMENT ERRORS: STATIC AND SENSOR CONTROL
Gravimetric volume measurement is sensitive to electrostatic charging, particularly when working with glass containers and in low-humidity environments. Static effects can introduce significant weighing errors, especially during long injection times or when measuring small volumes. Optimised weighing accessories and controlled test environments are therefore critical to achieving reproducible results.
Equally important is sensor reliability. Daily sensor checks, guided by software workflows, can help to detect issues before test series begin. This preventive approach avoids costly re-testing and protects the integrity of large datasets generated during verification or release testing.

Figure 2: Testing autoinjectors without medication.
TESTING AUTOINJECTORS WITHOUT MEDICATION
Some of the medications used in autoinjectors are very expensive, which also makes functional testing with medication very costly. A cost-effective alternative is to test the mechanical function of the injector before filling it with medication (Figure 2). In order to perform such tests under realistic conditions, an electromechanical servo actuator can be used in the testing machine. This simulates the counterforce of the medication cartridge, which normally acts on the autoinjector mechanism.
For automated functional testing, the autoinjector is positioned in the testing machine and started using advanced, intuitive testing software, such as ZwickRoell’s testXpert. The test then runs automatically within the testing machine. The result is a measurement of the force or torque required to remove the safety cap, as well as the spring rate of the built-in drive unit. Monitoring and calculation of results are also performed in the testXpert testing software, which also offers a fully integrated US FDA Part 11 option. To achieve higher throughput rates, this system can be equipped with a lightweight roboTest N handling system from ZwickRoell.
FULLY AUTOMATED AUTOINJECTOR TESTING
When testing insulin pens or pen injectors, a time- and resource-efficient test process is often necessary. A materials testing machine (optionally available with integrated torsion drive), combined with an automated testing system, can help to ensure reliable and cost-effective test results (Figure 3). The testing assistant roboTest N supports the user with simple applications. With one magazine filling, a user can typically automatically test 40 insulin pens or autoinjectors. The roboTest N can be easily adapted to changing test requirements with a high level of flexibility without requiring special programming knowledge.

Figure 3: A fully automated testing system for autoinjectors.
The robotic testing system roboTest R allows users to run fully automated tests on different functions of an insulin pen. It can, for example, measure the dosage setting, actuation force, glide force and specified dosage in one continuous process. It is possible to modify and combine the test methods of both test axes to suit specific testing needs.
“THE TESTXPERT TESTING SOFTWARE, WITH ITS EXPANDED TRACEABILITY OPTION SUPPORTING FDA 21 CFR PART 11, MAKES IT POSSIBLE TO GENERATE COMPLETE, TAMPER-PROOF DOCUMENTATION FOR THE ENTIRE TESTING PROCESS.”
Powered by ZwickRoell’s autoEdition3 automation software, the robot removes insulin pens from the magazine, feeds them into the testing machine and starts the test automatically. This reduces the risk of operator influence and helps to ensure accurate, consistent results. It also significantly boosts efficiency by increasing specimen throughput. It is also possible to manually test specimens at any time. The testXpert testing software, with its expanded traceability option supporting FDA 21 CFR Part 11, makes it possible to generate complete, tamper-proof documentation for the entire testing process.
EXTENDING TESTING TO ON-BODY DELIVERY SYSTEMS
Beyond classic spring-driven autoinjectors, the industry is seeing rapid growth in on-body delivery systems (OBDSs), also referred to as wearable, portable or variable injectors. These devices address the need to deliver larger volumes of high-viscosity biologics over extended periods, ranging from minutes to hours.
Testing OBDSs according to ISO 11608-6 introduces additional challenges, including long-duration volume measurement, evaporation control, prevention of crystallisation at the needle tip and interpretation of device feedback signals, such as LEDs or acoustic cues (Figure 4). Modular test platforms that combine peel testing, functional testing and camera-based monitoring enable comprehensive evaluation of these systems under realistic conditions.
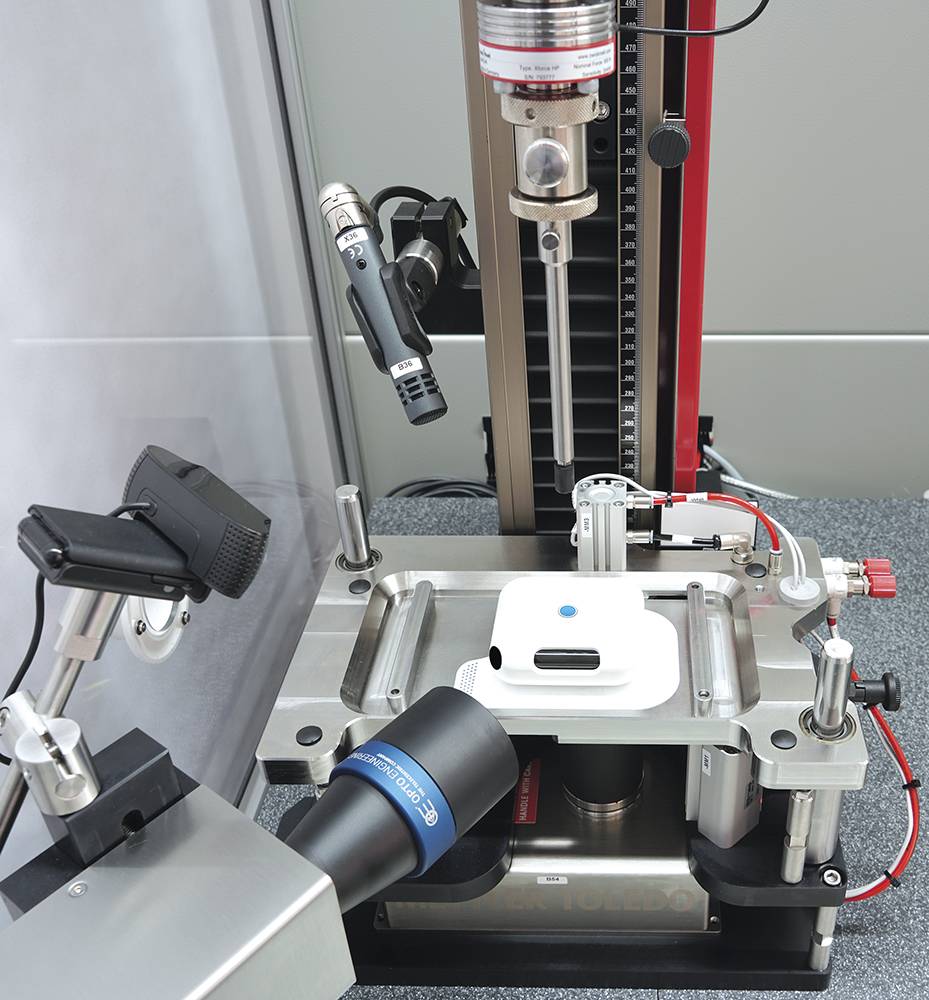
Figure 4: Testing system for OBDSs according to ISO 11608-6.

