To Issue 178
Citation: Khanolkar A, Vilaplana M, “A Configurable Patient-Centric Delivery Platform for Biologics and High-Concentration Formulations”. ONdrugDelivery, Issue 178 (Oct 2025), pp 42–47.
Asmita Khanolkar and Marta Vilaplana explore key challenges in delivering complex biologics and formulations, as well as the importance of early device involvement in preclinical development. They go on to consider how configurable platform technologies can support delivery of biologics, suspensions and high-viscosity formulations.
The development of novel therapies and biologics increasingly depends on optimising both formulation and drug delivery to ensure therapeutic efficacy. Achieving consistent pharmacokinetics and a positive patient experience is critical for successful clinical outcomes. However, delivery poses significant challenges: biologics and other advanced formulations often require high concentrations, large doses, greater volumes and higher viscosities, while also being fragile and stability-sensitive throughout manufacturing, storage and administration.
The delivery device plays a central role – without the right enabling technology, drug optimisation remains incomplete. Beyond technical performance, considerations such as delivery time, patient comfort and tolerance are essential for patient-centric solutions.
CHALLENGES IN DELIVERING COMPLEX BIOLOGICS AND FORMULATIONS
Optimising drug delivery requires balancing multiple considerations across formulation, device integration and patient experience. From a pharmacology perspective, understanding pharmacokinetic and pharmacodynamic (PK/PD) performance is critical, while, on the patient side, factors such as tolerability, optimal dose, concentration and viscosity must be addressed. Achieving consistent pharmacokinetics relies heavily on understanding the formulation behaviour and optimising delivery for dependable results. Complex formulations such as non-Newtonian fluids, high-viscosity biologics and suspensions demand thorough pre-characterisation to determine delivery feasibility and guide the selection of the most effective device pathway (Figure 1).1
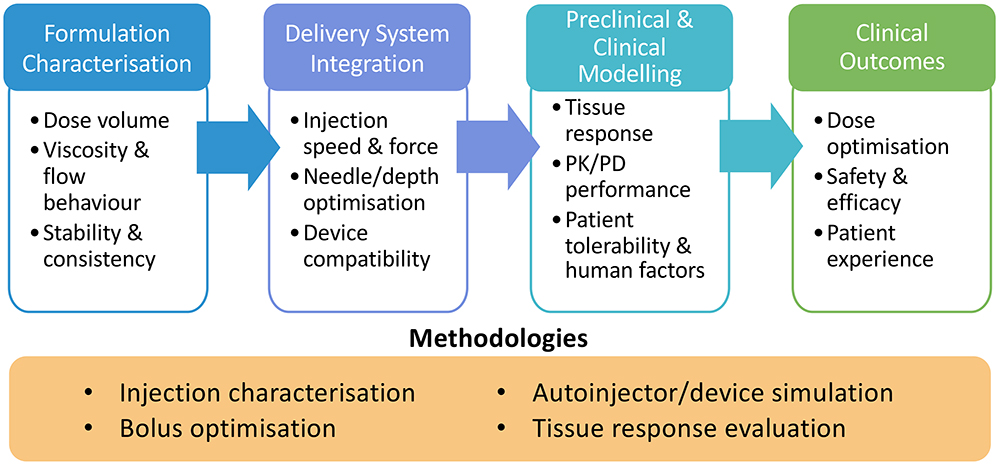
Figure 1: A continuum of optimisation – from formulation characterisation to clinical outcomes – showing how early device integration and systematic methodologies enable effective delivery of complex formulations.
The Role of an Enabling Delivery Device Platform
The shift from infusion-based therapies to rapid, subcutaneous (SC) delivery is reshaping patient care, enabling treatment to move from hospital to home. This transition offers clear benefits – greater convenience, reduced clinic time and improved quality of life – but it also requires careful adaptation of formulations from intravenous (IV) to SC use. Achieving therapeutic efficacy in this context demands reconfiguration of dose, concentration and viscosity, often resulting in more challenging formulations.
In this context, the delivery device platform becomes central. An enabling platform provides the flexibility to accommodate a range of variables: dose volumes, needle sizes, container formats, formulation stability and acceptable delivery times from a patient perspective. Integrating such a platform early in development allows iterative preclinical evaluation, reduces risk and accelerates the path to viable in-home therapies. In essence, device platforms are not just supporting technologies – they are key enablers of the transition towards patient-centric, advanced biologic delivery (Figure 2).
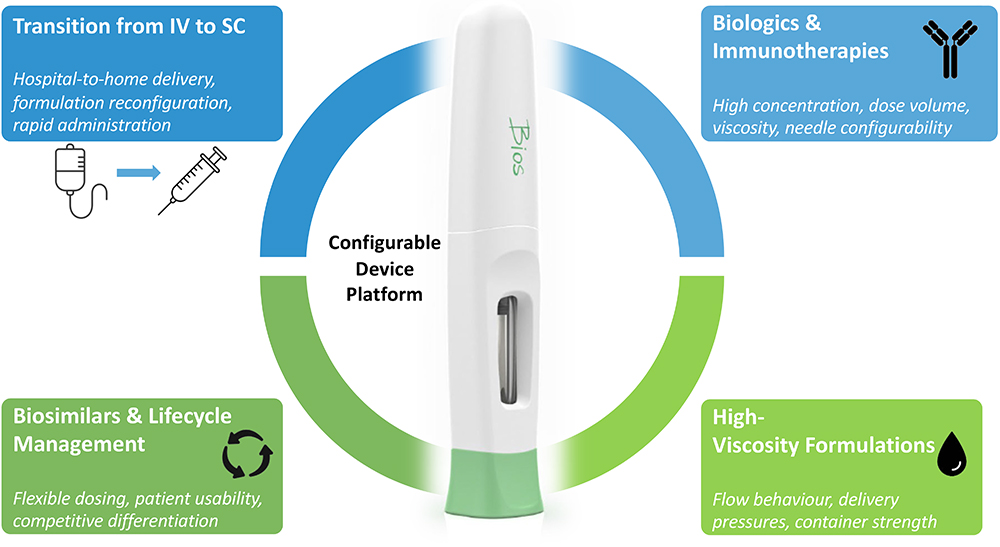
Figure 2: Device platforms act as a configurable foundation, addressing unique delivery challenges while ensuring patient usability and consistency.
The need is even more pronounced in biologics and immunotherapies, which push the limits on dose size and delivery volume. In line with high-viscosity requirements, developing high-pressure systems and container choices that withstand the mechanical stresses of delivering such formulations becomes a necessary challenge, with biosimilars adding further demand for scalable, adaptable solutions. Suspensions and non-Newtonian fluids amplify the complexity, requiring device platforms capable of consistent performance across a wide range of environments.
Additionally, the rise of precision medicine and orphan drugs highlights the importance of plausible and cost-effective solutions for smaller patient populations with diverse dosing requirements. Success depends not only on technical delivery but also on patient adoption, which is driven by ease of use, comfort and trust in the device.
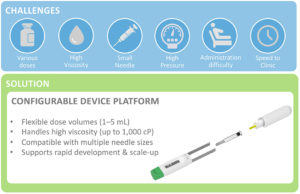
Figure 3: Configurable device platforms bridge the gap between delivery challenges and real-world solutions that accelerate development and enable patient-centric care.
Ultimately, a configurable device platform – built on modular components such as powerpacks, containers and needles – provides the foundation for delivery optimisation from the preclinical stage through to real-world patient use. Far more than a support tool, such platforms are enablers of therapeutic success, bridging the gap between complex formulations and patient-centred outcomes (Figure 3).
“BY INTEGRATING DELIVERY CONSIDERATIONS UPSTREAM,
THE PATH TO A VIABLE, PATIENT-READY THERAPY BECOMES MORE PREDICTABLE AND EFFICIENT.”
EARLY DEVICE OPTIONS FOR DELIVERY OPTIMISATION
In the preclinical stage, formulators face the dual challenge of optimising drug formulations while anticipating the realities of delivery. Early involvement of device options provides critical insights into syringe suitability, formulation injectability, delivery consistency and achievable injection times. By integrating delivery considerations upstream, the path to a viable, patient-ready therapy becomes more predictable and efficient.
Injection Characterisation of Complex Formulations
The first step in delivery optimisation is to characterise how a formulation behaves under actual injection conditions. An injection characterisation system enables empirical mapping of flow behaviour by measuring delivery pressure during injection. Because many biologics and suspensions exhibit non-Newtonian flow behaviour, multiple test iterations are required to assess viscosity stability under varying shear rates and temperatures. These tests generate large datasets (>10,000 points per run) across a range of needle sizes, forces and flow rates, making shear sensitivity more apparent. The resulting viscosity values can then be used to predict injection times and inform device requirements (Figure 4).
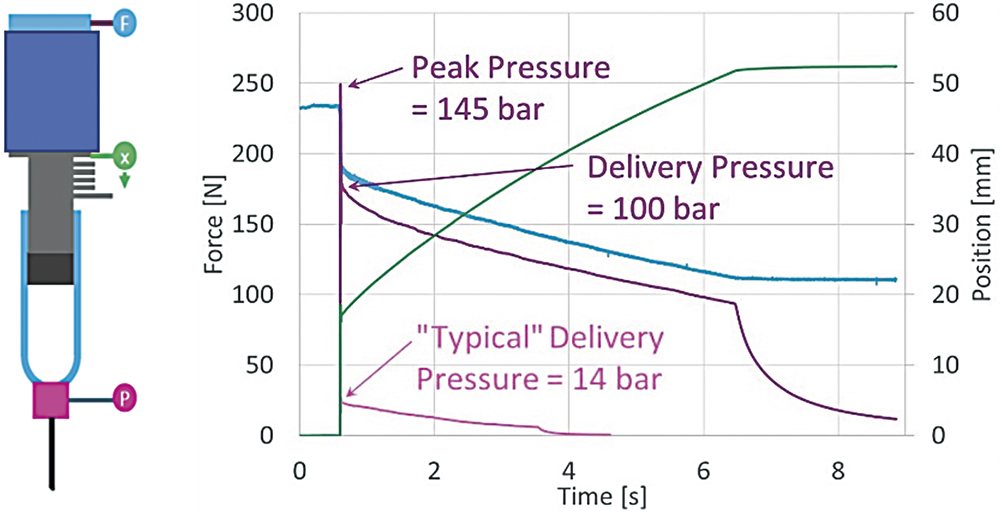
Figure 4: An injection characterisation system can map key delivery parameters e.g. force (F), pressure (P) and stroke (x) to inform predictions of injection times
for non-Newtonian formulations.
For non-Newtonian fluids, behaviour is further quantified using the power law model, where fluid mobility (K) and shear-thinning/thickening properties (n) define the relationship between shear stress and shear rate. These parameters establish a mathematical model that links formulation behaviour to delivery feasibility.
Shear Stress = K × Shear Raten
Autoinjector Demonstration for Empirical Performance
Data from injection characterisation are translated into empirical device testing. A demonstrator system – mirroring the design and power source of the candidate autoinjector – validates whether predicted injection times align with real-world delivery performance. Using compressed gas to drive the plunger, the demonstrator system can accommodate a range of syringes, fill volumes and canister pressures. This allows comprehensive evaluation of delivery outcomes, including injection time ranges, residual volume and consistency across variables, demonstrating the capability of the device and providing an understanding of the expected data spread derived from the input variables. Such testing also highlights container limitations, particularly under high-viscosity conditions. The structural strength of the syringe (e.g. glass versus polymeric) may dictate safe operating pressures, requiring alignment between formulation, device power and container material to ensure reliable and safe injections.
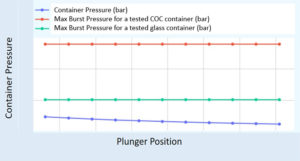
Figure 5: Syringe structural performance quantified in maximum burst pressure – tested to failure, relative to the required pressure within the syringe barrel for
high-viscosity applications.
Figure 5 shows results from a study comparing syringe structural performance depending on material. The insights gained from injection characterisation and empirical testing enable an informed path towards device configuration. By varying powerpack strength (gas pressure), container type, needle size and dose volume, a combination can be identified that delivers optimal performance for formulations of differing concentrations and viscosities. This iterative process underpins the role of a configurable device platform, ensuring that delivery challenges are addressed early and that the final result balances formulation requirements, technical feasibility and patient experience (Figure 6).
Figure 6: Path to device-formulation optimisation: iterative testing and device configurability enable robust delivery solutions aligned with formulation needs and patient experience.
“THE BIOS PLATFORM HAS BEEN PURPOSEFULLY DESIGNED TO
ADDRESS THE FULL SPECTRUM OF CHALLENGES OUTLINED ABOVE – FROM THE SHIFT OF INFUSION THERAPIES TO RAPID SC DELIVERY, TO THE COMPLEXITIES OF BIOLOGICS, BIOSIMILARS AND HIGH-VISCOSITY FORMULATIONS.”
THE BIOS PLATFORM DESIGN: AN ENABLING PLATFORM SOLUTION
The Bios platform has been purposefully designed to address the full spectrum of challenges outlined above – from the shift of infusion therapies to rapid SC delivery, to the complexities of biologics, biosimilars and high-viscosity formulations. As an adaptable, gas-powered autoinjector, it supports a wide range of volumes (0.5–5 mL) and viscosities (up to 1,000 cP) while remaining compatible with ISO 11040 staked-needle prefilled syringes. Its configurable architecture allows early integration into development and aligning of the device design with formulation properties from the outset.
Patient Centric by Design
The Bios device is built around the patient experience. With intuitive two-step operation, a large viewing window to track progress, hidden needle for comfort and audible start/end clicks, it is accessible even to patients with dexterous or visual limitations. These features not only improve usability and adherence but also support the hospital-to-home transition by making complex treatments simpler and safer in self-administration settings (Figure 7).
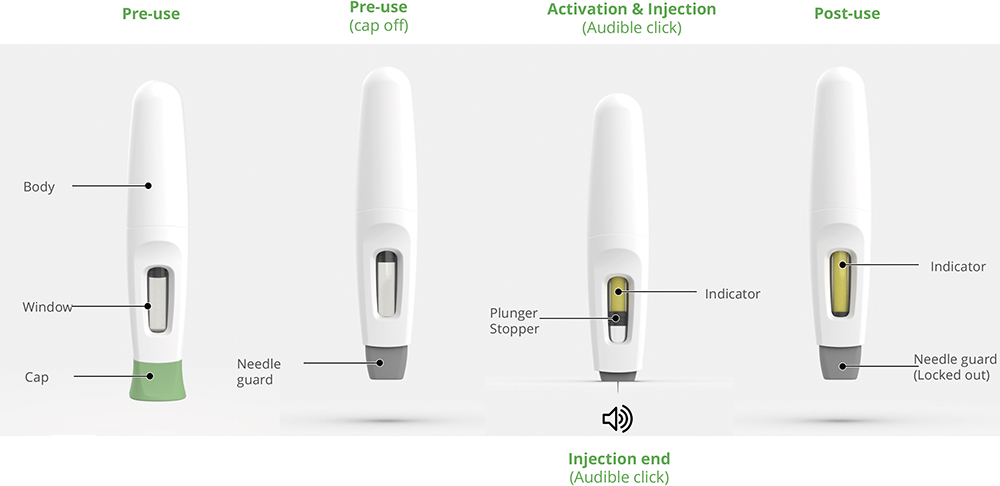
Figure 7: The Bios platform – an intuitive two-step solution, configurable and suited to patient requirements.
Accelerating Delivery Optimisation
By combining configurability with empirical validation of injection performance, the Bios platform creates a bridge between preclinical formulation development and clinical readiness. Its ability to accommodate a wide variety of container types, volumes, and flow behaviours makes it a versatile solution for biologics, biosimilars and orphan drugs alike. In doing so, it enables earlier, data-driven device integration, reduces risk in development and helps to accelerate speed-to-clinic.
REFERENCES
- Khanolkar A, White S, Margerison E, “A Novel Method to Optimize Drug Delivery for Parenteral Products Involving New Therapies and Unmet Needs”. Pharm Res, 2023, Vol 40(10), pp 2303–2315.

