To Issue 158
Citation: Regard A, Audibert R, Chandra A, “Addressing CNS Therapies Through Nose-to-Brain Pathways”. ONdrugDelivery, Issue 158 (Apr 2024), pp 73–76.
Alain Regard, Raphaële Audibert and Audrey Chandra, discuss the advantages of nasal delivery and highlight the challenges of the nose-to-brain pathways.
Injectable forms, including complex molecules such as biologics, have been the standard go-to therapies for decades. However, the overall industry paradigm is shifting to less-invasive delivery routes to allow self-administration in a homecare setting, a shift that occasionally necessitates drug repurposing.
“Recently, there has been growing interest and further exploration in nasal delivery for systemic-acting drugs by targeting nasal turbinates.”
NASAL DELIVERY FOR BETTER PATIENT ADHERENCE AND COMPLIANCE
The nasal cavities are highly vascularised and innervated anatomical structures, which present an interesting opportunity to deliver rapid onset therapies with low toxicity, thereby minimising adverse events. Nasal delivery allows non-invasive local and systemic treatment administration and requires no intervention from healthcare professionals. Nasal administration offers superior bioavailability by bypassing the hepatic first-pass effect often encountered with oral medication.
Nasal devices are needle-free, which fosters patient acceptance, leading to positive therapy outcomes following improved patient adherence and compliance.
Topical therapies still dominate the nasal market to treat allergic rhinitis, sinusitis and nasal congestion. Allergic rhinitis is mostly seasonal and usually relieved by nasal sprays containing topical-acting medications (Figure 1).
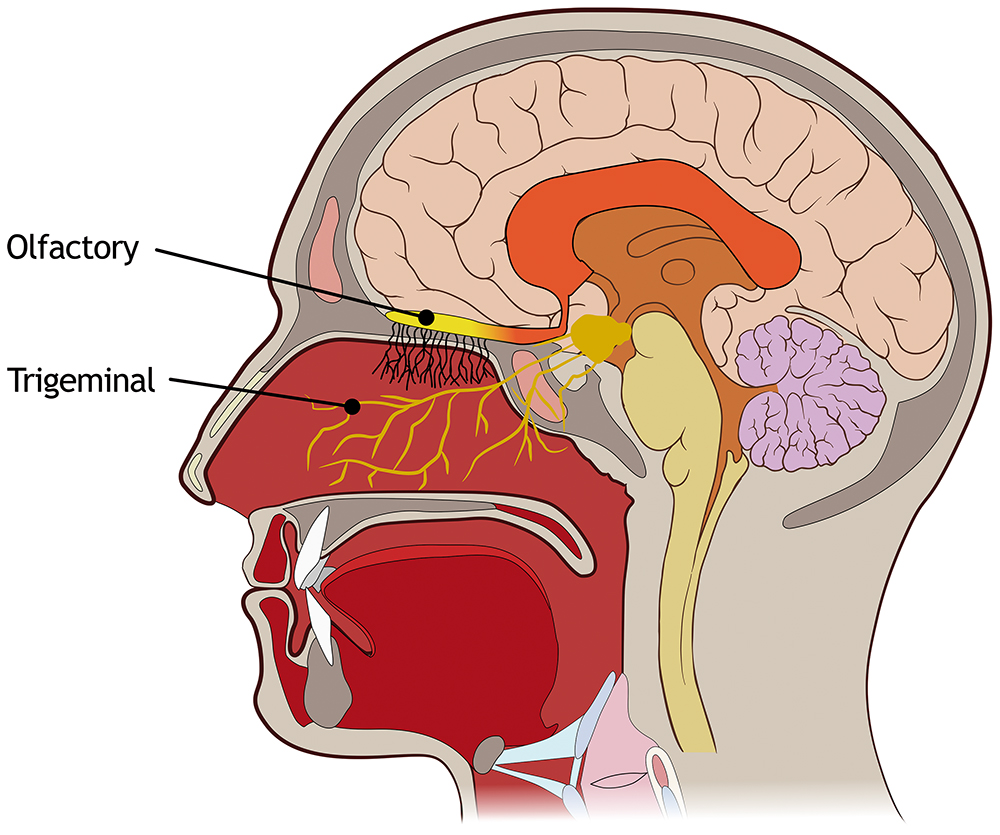
Figure 1: Nasal cavity zone of interest to address systemic and direct nose-to-brain delivery.
SYSTEMIC AND DIRECT NOSE-TO-BRAIN ADMINISTRATION
Recently, there has been growing interest and further exploration in nasal delivery for systemic-acting drugs by targeting nasal turbinates. Highly vascularised turbinates occupy a large surface area of the nasal mucosa, offering a convenient pathway for systemic delivery. Several drug pipelines are targeting diverse therapeutic areas, driving demand for marketed products, particularly in emergency and crisis contexts, often using unit-dose sprays.
“Further studies are needed to fully characterise the deposition performance of such devices while considering the wide interpatient variability of the nasal anatomy.”
Another promising route currently under exploration is the nose-to-brain pathway, which presents opportunities for efficient drug delivery, particularly for central nervous system (CNS) therapies. Traditional treatments for brain disorders are often very invasive, such as subcutaneous or intravenous injections in a medicalised environment targeting the brain and spine. These procedures are often linked with significant side effects adversely affecting patients’ quality of life.
Some CNS therapies are currently administered intranasally to treat potent drug overdoses or migraines (such as naloxone, zolmitriptan, etc) and distributed to the brain via the blood after absorption in the turbinates. Anatomically, the brain is protected by the blood-brain barrier (BBB), which prevents > 95% of molecules entering the CNS through the bloodstream. The ability of certain drugs to permeate the BBB is impeded by their physicochemical properties and only affects small molecules. A limited number of drug candidates can, therefore, enter the brain by crossing the BBB.
Direct intranasal brain delivery avoids the BBB and requires lower doses. This approach reduces toxicity and minimises off-target effects as the medications mitigate the first-pass metabolism. This path may also allow the delivery of large molecules into the brain. Targeting olfactory and trigeminal nerves through the nasal cavity may be the best route for direct brain delivery. Olfactory nerves are the preferred targeted site as they are concentrated in a small area. Trigeminal nerves are spread over the cavity, and targeting them would mean coating the entire nasal cavity with the formulation, leading to the risk of systemic absorption.
DIRECT NOSE-TO-BRAIN PATHWAY CHALLENGES
The nose physiology should be considered when developing drug formulations for the direct nose-to-brain pathway as challenges may be encountered:
- Formulation Development: The limited volume of formulation that can be applied to the nose, mucociliary clearance, the presence of the mucosal layer and local enzymes are some of the factors that can hamper drug absorption through the intranasal route. Metabolic enzymes present in the olfactory mucosa must also be considered when designing a formulation for the nose-to-brain route. Consequently, intranasal formulations must be composed of biocompatible and odourless excipients and avoid rapid elimination due to mucociliary clearance and/or enzymatic degradation. Furthermore, they must present appropriate viscosity, physiological tonicity and a pH compatible with the nasal mucosa.1,2 Thus, different strategies have been investigated to overcome the challenges of this route of administration. Most of these approaches aimed to enhance molecular absorption and permeability by increasing the time in which the dosage form remains in the nasal mucosa and promoting drug concentration in the CNS.3 These strategies include the use of permeation and absorption enhancers, cell-penetrating molecules, mucoadhesive and mucopenetrating agents, enzyme inhibitors, hydrogel systems, nanoparticulate drug delivery systems or a combination of different strategies.
- Anatomy Gaps and Variability: In addition to formulation challenges, major anatomical gaps between animal models used in preclinical studies and real human anatomy raise further challenges to mimic relative size, surface–volume ratio and the extent of the innervation network for relevant animal-to-human translation. Nasal cavity anatomy is subject to interpatient variability , with additional contributing factors such an ethnicity, gender and age. Structural or inflammatory nasal pathologies can also generate some obstruction, and it is important to consider these possibilities when developing and testing new treatments.
- Olfactory Cleft Target and Drug Efficacy Quantification: The olfactory region represents a small surface area, around 5 cm2 (3,000 cm2 including the microvilli), and is located very deep into the nose, which is extremely difficult to reach with conventional nasal spray devices.4 Monitoring pharmacokinetics in the bloodstream is relatively straightforward; however, quantifying the amount of drug reaching the cerebrospinal fluid or brain tissues in humans remains a challenge.

Figure 2: Liquid jet dosage form to reach olfactory cleft.
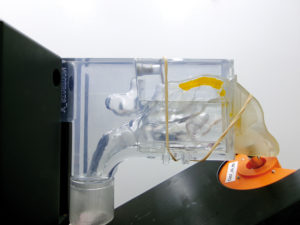
Figure 3: Typical deposition pattern observed on Nemera’s Athena nasal cast.
JET-BASED NASAL DEVICE FOR NOSE-TO-BRAIN DELIVERY
Nemera is developing a jet-based nasal device that addresses several of the aforementioned challenges. The jet nozzle has been designed to release a narrow and steady liquid flow, as shown in Figure 2.
The tested jet nozzle prototype configurations show promise in delivering liquid formulations to the olfactory area in a targeted manner. Several design configurations have demonstrated high deposition rates in the olfactory area, exceeding 50% on two different nasal casts (Figure 3).
In addition, further studies are needed to fully characterise the deposition performance of such devices while considering the wide interpatient variability of the nasal anatomy. Studying the user/patient interface to ensure the correct usage steps for drug efficacy is essential. For instance, designing an easy-to-use device to reduce administration variability includes nozzle tip insertion depth, orientation of the device, patient position during administration, etc.
CONCLUSION
Treating CNS pathologies via non-invasive routes, such as the direct nose-to-brain pathway, offers a promising solution from the patient’s perspective as it can reduce the discomfort associated with traditional injection therapies while also limiting the systemic side effects.
Researchers are continuously looking into opportunities to develop both a device and adequate formulations that may target the olfactory cleft to demonstrate drug efficacy and treatment feasibility.
Nemera’s long-standing expertise in developing and manufacturing complex nasal devices encourages the company to continue its efforts in making the drug delivery device solutions of tomorrow better. Nemera believes it is critical to interact with industry leaders and peers to ensure the success of its innovations. It is essential to collaborate and reflect to achieve solid outcomes with neurology and ear, nose and throat (ENT) specialists for complex novel therapies, such as nose-to-brain delivery.5
Nemera specialises in streamlining the integration of the customer’s selected device and drug product to accelerate the development of the combination product and speed to market. Nemera’s comprehensive services cover a wide range of critical areas including:
- Analytical services and design verification: customised laboratory and testing services for a specific device.
- Human factors and user experience management: safe and effective devices surrounded by assets to drive engagement.
- Regulatory strategy and submission authoring: accelerating the path to approval.
- Drug–device assembly and packaging support: managing the combination product.
Nemera’s expertise enables pharma companies to confidently navigate the complexities of a combination product knowing there is a trusted partner by their side to accelerate the process.
With long-standing experience in developing and manufacturing an extensive range of multidose nasal spray pumps, commercialised worldwide for diverse therapeutic areas and applications, Nemera is the ideal partner for direct nose-to-brain projects. To address systemic-acting drugs with a unit-dose spray, the company’s UniSpray system is now commercially available and targets emergency and crisis treatments.
To find out more about nasal drug delivery and Nemera’s offering in this space, visit: www.nemera.net/our-products/ear-nose-throat.
REFERENCES
- Costa CP et al, “Intranasal delivery of nanostructured lipid carriers, solid lipid nanoparticles and nanoemulsions: a current overview of in vivo studies”. Acta Pharm Sin B, 2021, Vol 11(4), pp 925–940.
- Costa C et al, “Nose-to-brain delivery of lipid-based nanosystems for epileptic seizures and anxiety crisis”. J Control Release, 2019, Vol 295, pp 187–200.
- Gao H, “Progress and perspectives on targeting nanoparticles for brain drug delivery”. Acta Pharm Sin B, 2016, Vol 6, pp 268–286.
- Gizurarson S, “Anatomical and histological factors affecting intranasal drug and vaccine deliver”. Curr Drug Deliv, 2012, Vol 9(6), pp 566–582.
- Radulesco T, Serrano E, Michel J, “The Role of Ear, Nose, and Throat Specialists in the Nose-to-Brain Pathway”. JAMA Otolaryngol Head Neck Surg, 2023, Vol 149(9), pp 769–770.

