To Issue 141
Citation: Cuevas Brun EH, Hsu Y-M, “Advancements in Monitoring Adherence – a Smart Mesh Nebuliser”. ONdrugDelivery, Issue 141 (Dec 2022), pp 22–26.
Edgar Hernan Cuevas Brun and Yuan-Ming Hsu consider patient adherence and the role HCmed Innovations’ AdheResp mesh nebuliser plays in monitoring adherence.
“Digital approaches have advanced to the extent that some of the services that were effective in the pandemic remain in use today and are expected to last in the post-pandemic world.”
Patient adherence for people who suffer from respiratory conditions is essential to improve and/or stabilise medical conditions and symptoms, especially with chronic diseases such as asthma and allergic rhinitis.1 Moreover, patient adherence has been investigated in a group of interventional studies that revealed its benefits for several conditions, including chronic obstructive pulmonary disease (COPD) and cystic fibrosis.2
It has been identified that, with respiratory diseases, adherence can be considered as an approach to reduce cost by keeping patients’ conditions under control. To enhance adherence and compliance, advancements in technology over the past decade have vastly contributed to the development of mobile apps and smart devices that can be effective tools to facilitate monitoring and tracking patients’ behaviour and support their treatment adherence.
As an extension of the advancements, in 2018 during the 71st World Health Assembly, the WHO stated that mobile health (mHealth) apps are an essential part of digital health and that access to mobile wireless technologies could provide significant health service resources as its use is far-reaching.3 In the same year, it was also reported that several mobile apps focused on the management of chronic respiratory conditions were available in the two major mobile app distribution platforms: Apple App Store and Google Play. Among the apps, most were intended for asthma management but also included COPD, rhinitis and rhinosinusitis. Their scope centred around three areas: self-monitoring, personalised feedback and user education.4 These areas are fundamental to the support of patient adherence.
During the covid-19 pandemic, digital healthcare undoubtedly became one of the solutions to provide healthcare services, accelerating its spread and adoption. Digital approaches have advanced to the extent that some of the services that were effective in the pandemic remain in use today and are expected to last in the post-pandemic world.5 This situation has further advanced the use of smart devices and mHealth apps in all fields, including adherence monitoring in the respiratory field.
“Relying on the detection of pressure change during a breathing cycle, the AdheResp nebuliser can trigger aerosol generation during a specific section of the inhalation phase.”
DIGITAL TECHNOLOGIES FOR INHALATION THERAPY
Several smart cap accessories for inhalers have been developed in the last few years, with some already being marketed as tools to monitor patient adherence. Many of these devices are designed to be incorporated into pressurised metered dose inhalers, offering visual, acoustic or haptic feedback during the treatment intake and recording the number of dosages being taken and the time of administration. Their use is commonly involved in the delivery of bronchodilators and inhaled corticosteroids.
Data from the smart caps is mostly transmitted to a mobile device that can be synchronised later to a health cloud, which allows medical practitioners and patients to access the information as needed. From a large number of available smart inhalers and mHealth apps for asthma treatment, one study identified six apps, with two being used in seven studies. The results of the studies showed that the interventions moderately improved inhaler adherence; however, there were no significant improvements in asthma control test results.6
Connectivity via Bluetooth has been incorporated in some nebulisers to monitor adherence for treatments involving antibiotics and vasodilators. Data transmission of time stamps and personalised treatment reminders are some of the key features in these devices to improve patient adherence and allow a more reliable source to collect treatment history data for medical evaluation and the prognosis following treatment. An increase in the number of nebulisers of this type is expected in the coming years as digital approaches continue to tap into the inhalation therapy field.
A NEW SMART BREATH-ACTUATED NEBULISER
The AdheResp smart breath-actuated mesh nebuliser has been designed and developed by HCmed Innovations to be used in combination with formulations that require a high level of delivery efficiency while reducing fugitive aerosol emission. Relying on the detection of pressure change during a breathing cycle, the AdheResp nebuliser can trigger aerosol generation during a specific section of the inhalation phase. This feature is particularly useful when aerosolising formulations, such as antibiotics and biologics, which require reducing drug waste during treatment to improve efficiency and, at the same time, protect bystanders from inhaling fugitive aerosol.
Besides breath actuation, another major feature of the AdheResp nebuliser is Bluetooth connectivity, which has been developed to offer an effective vehicle as an adherence monitoring platform for different diseases. The AdheResp nebuliser has a Bluetooth module integrated into the main unit of the device and allows the transfer of three sets of data: time stamp, pressure reading and battery level. The data is continuously shared from the nebuliser to a mobile device that acts as a receiver to display information for the patient and/or medical practitioner. Connection to a healthcare cloud could be further achieved with the implementation of a function from mHealth apps.
“To demonstrate the capabilities of the connectivity feature, HCmed is developing a demonstration mHealth app that uses the transferable data from the AdheResp nebuliser.”
AdheResp: Data Transmission
To demonstrate the capabilities of the connectivity feature, HCmed is developing a demonstration mHealth app that uses the transferable data from the AdheResp nebuliser. The types of data that can be transferred to a mobile device are described as follows:
Time Stamp
The total treatment length – and times at the beginning and end of each treatment – can be displayed from the time stamps collected during nebulisation. As well as benefiting the patients themselves, these values are useful for clinicians and medical practitioners to monitor patient adherence.
Pressure Reading
The pressure readings throughout the treatment are transmitted from the device to the mobile device. The data can be used to profile the pressure change created by the breathing pattern of a patient with wave graphs.
Battery Level
Levels of battery power are transmitted when the device is first connected to allow patients to have a better understanding of the battery status.
Figure 1: AdheResp – demonstration mobile app sign-up/log-in page.
mHealth Demo: Interface
The demonstration mobile app for the AdheResp nebuliser has been designed to provide an overview of a standard interface, rather than focusing on a specific indication. As AdheResp is a nebuliser for the development of combination products, each indication is expected to make use of the transferable data in different ways; therefore, a simpler approach was used for the demonstration mobile app.
The initial page requires users to sign up upon first use to complete the registration process or log in (Figure 1). Basic personal information has to be input at this stage to create an account with its corresponding password to secure patients’ data.
A total of four pages can be navigated through in the AdheResp demonstration app: AdheResp, Treatment, Knowledge and Settings. Each one of the pages displays essential information for the user to self-educate, operate the device and monitor their treatment by means of the collected data.
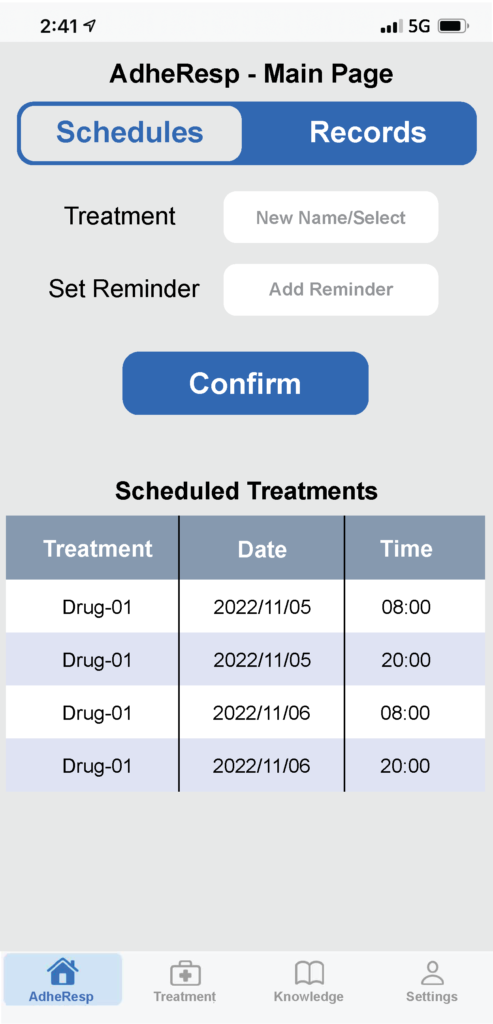
Figure 2: Mobile app main page – schedules.
AdheResp: Main Page
Once registration is complete, the user will be directed to the AdheResp main page section where the scheduled treatments and reminders are shown. This section also allows users to schedule new treatments (Figure 2). Furthermore, the main page offers the option of accessing records from previous treatment for which the treatment and timeframe can be first selected to obtain information about the date and time of treatment, duration and pressure profile (Figure 3A). This last feature provides an extended graph of the changes in pressure during the treatment that follows the breathing pattern of the patient, providing valuable data for posterior evaluations (Figure 3B).
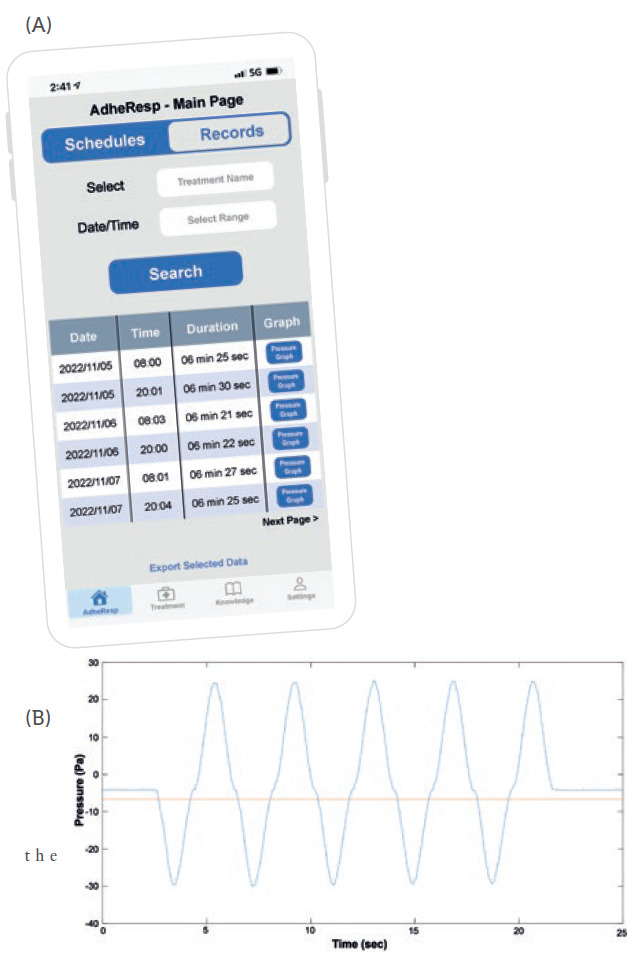
Figure 3: (A) Mobile app main page – records. (B) Pressure change graph generated from a breathing pattern.
Treatment
The Treatment page offers three options for the user to select: start treatment, transfer data and healthcare cloud. The start treatment option guides the patient to prepare for treatment, from the medication loading to Bluetooth pairing and the nebulisation process (Figure 4). While nebulising, a graphics interchange format guides the user to inhale and exhale at a specific rhythm to enhance aerosol intake. Data are continuously transferred to the mobile app during this process until the treatment is completed. At this point, the AdheResp nebuliser will automatically switch off and the mobile app will be delinked.
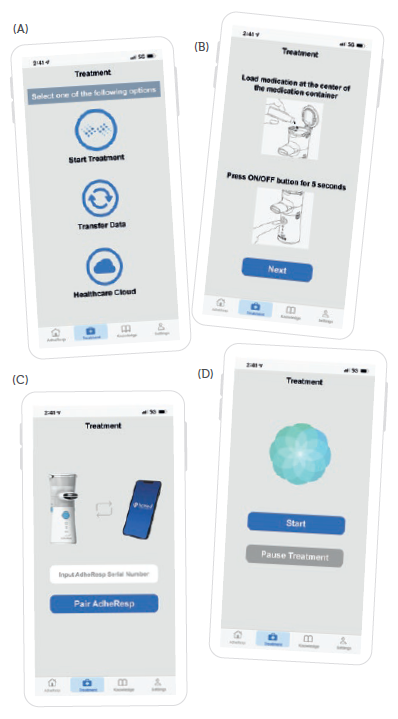
Figure 4: (A) Start treatment interface: (B) treatment preparation, (C) Bluetooth pairing and (D) treatment in progress.
“Different countries and regions have adopted their particular approaches on how to regulate connected devices and mHealth apps.”
Transferring data from previous treatments when the nebuliser was not connected to a mobile device is possible with the second option of the Treatment page, called transfer data. The AdheResp nebuliser is able to store data for up to 30 treatments, before overwriting previous ones. This function can be used when the patient is charging the nebuliser and the Bluetooth function is in pairing mode. At this moment, the patient can press the icon to transfer data, and all the sets of data that had not been transferred from the nebuliser previously will be sent at pairing.
Last but not least, the healthcare cloud offers the option to upload data stored in the mobile device to a secure cloud. The cloud access could then be configured to give access to healthcare practitioners and caregivers to observe and monitor the treatment data of the patient, who should give consent prior to data collection.
Knowledge
The Knowledge page presents a space for users to learn about the basic use and maintenance of the product. It is currently being built to combine text, images and videos that can describe the correct handling of the AdheResp nebuliser to ensure optimal performance. The section also includes a troubleshooting area and a space for information about the drug expected to be administered and the indication being treated by the combination product to provide educational information to patients about their condition and special care.
Settings
Modifications and updates to personal information and passwords are available in the Settings page. This page is also expected to offer functions such as printing treatment summary reports, clearing stored data and even scheduling future appointments with a clinician to follow up treatment progress.
For combination development, data gathered by HCmed has pointed to the conclusion that most pharmaceutical companies prefer to develop their own mHealth app as each indication may require some customised interfaces, features and parameters. Considering this, the development of the demonstration app showcases the key functions that can be created around the transferable data capabilities of the AdheResp nebuliser, providing a solid reference for other potential developments.
Future Challenges in mHealth
Although it is undeniable to say that the future of healthcare may be in the hands of digital healthcare and connectivity, there are still several aspects to be further refined, such as data safety, efficacy and patient privacy. Different countries and regions have adopted their particular approaches on how to regulate connected devices and mHealth apps. The US FDA has updated the Device Software Functions Including Mobile Medical Applications guidance to encourage and regulate mHealth apps, while the Health Information Technology for Economic and Clinical Health Act and the Health Insurance Portability and Accountability Act are in place to protect patient information for data transmitted from medical devices. On the other hand, the EU has also played its part with the European Data Protection Board, which ensures the safeguarding of users’ data according to the General Data Protection Regulation applicable to mHealth Apps.7
With the continuous evolution of digital healthcare, addressing existing issues related to cybersecurity and personal data information is crucial to build trust from users and speed up the spread of connected devices and mHealth apps. These two have the potential to be highly beneficial for adherence monitoring of respiratory diseases, such as asthma, COPD, pulmonary arterial hypertension and others. The incorporation of these solutions in combination products, using nebulisers like the AdheResp device, could open up several new possibilities for future treatments, providing better outcomes and cementing the adoption of digital health. Moreover, pharmaceutical companies,which are allowed to own and analyse the patient adherence database, could also benefit from the user preference to adjust existing and new medication regimes.
REFERENCES
- Baiardini I, Novakova S, Mihaicuta S et al, “Adherence to treatment in allergic respiratory diseases”. Expert Rev Respir Med, 2019, Vol 13(1), pp 53–62.
- Calthorpe RJ, Smith S, Gathercole K et al, “Using digital technology for home monitoring, adherence and self-management in cystic fibrosis: a state-of-the-art review”. Thorax, 2020, Vol 75(1), pp 72–77.
- “Seventy-first World Health Assembly: Geneva, 21-26 May 2018: summary records of committees, reports of committees”. World Health Organization webpage, accessed Nov 2022.
- Sleurs K, Seys SF, Bousquet J et al, “Mobile health tools for the management of chronic respiratory diseases”. Allergy, 2019, Vol 74(7), pp 1292–1306.
- Lee SM, Lee D, “Opportunities and challenges for contactless healthcare services in the post-COVID-19 Era”. Technol Forecast Soc Change, 2021, Vol 167, 120712.
- Nguyen E, Miao B, Pugliese N et al, “Systematic Review of mHealth Applications That Interface with Inhaler Sensors in Asthma”. J Allergy Clin Immunol Pract, 2021, Vol 9(2), pp 844–852.e3.
- Hassanaly P, Dufour JC, “Analysis of the Regulatory, Legal, and Medical Conditions for the Prescription of Mobile Health Applications in the United States, The European Union, and France”. Med Devices (Auckl), 2021, Vol 24(14), pp 389–409.

