Citation: Morgan V, “Altering Patient Treatment: How SC Delivery can Help Patients Manage Chronic Conditions”. ONdrugDelivery Magazine, Issue 97 (May 2019), pp 34-37.
Victoria Morgan looks at the benefits of combining an active pharmaceutical product with a novel subcutaneous delivery device, and highlights some of the partnerships West has with biopharmaceutical and other companies, which have led to market launches of products incorporating its on-body delivery system.
In recent years, the pharmaceutical industry has become steadily more patient centric. The impact can be seen in nearly every aspect of the industry – from regulatory guidance, trial design and drug delivery to new drugs proliferating the pipeline, such as biologics. Biologics are helping to revolutionise the treatment of chronic diseases such as multiple sclerosis and other autoimmune diseases – by helping patients take less frequent injections.
“There is a paradigm shift underway in terms of what is possible in a pain-tolerant larger-volume injection.”
Additionally, by targeting specific components of a disease in ways never thought possible before, these therapies may also help some acute conditions, including certain types of cancer, become manageable chronic conditions.A real focus of biologics research and development lies in new molecular entities which can be administered into the subcutaneous (SC) tissue. Therapeutic areas such as growth hormones and diabetes have long shown efficacy through SC delivery and an established patient acceptance of self-injection and pain tolerance. These therapeutic areas are rapidly being joined by oncology, and autoimmune and blood disorders, which traditionally had IV and infusion as the main routes of administration but which are now seeing novel drug launches with SC delivery routes (Figure 1).
THE SC SPACE IS NO LONGER THE NEW FRONTIER

Figure 1: Number of NME SC biologics programmes in the clinic.1
Administration of large-volume medicines has always been a challenge – one that has traditionally forced many drugs to be formulated into the <1 mL space. This was the approximate volume which would be tolerated by the patient while still being an efficacious dose. Yet there is a paradigm shift underway in terms of what is possible in a pain-tolerant larger-volume injection – all because of new ways of accessing the SC space. Whereas intravenous (IV) infusions usually have to be administered in a hospital or a doctor’s office, SC administration may be performed by a healthcare professional at the patient’s home or even by patient self-administration.2 In addition, subcutaneous administration helps to treat patients with poor venous access or to spare patients’ venous capital.3 SC administration is of particular benefit for long-term or chronic drug treatments. In addition, SC administration may be better tolerated, compared with IV administration, as the slow absorption may abrogate side effects related to high serum concentrations.
LET’S TALK SUBCUTANEOUS
For patients diagnosed with haemophilia A, a typical treatment regime would be octolog alfa every 48 hours, with numerous injections to treat on-demand bleeds. The total number of injections each week could easily be more than 10. Patients diagnosed with multiple sclerosis may relapse when adherence to therapeutic regimens wanes, forcing a 3–5 day hospital stay for IV steroids. While some diseases have moved the needle forward in terms of patient compliance – such as the regular use of insulin pens in diabetes – many people worldwide still suffer from the daily reminder and pain of their injections.
The SC space allows us to think differently about how a patient perceives his or her illness by allowing larger volumes to be administered less frequently. When patients are not frequently reminded of their condition, adherence can be improved. Pharmaceutical companies recognise this, as do regulators, as evidenced by the increasing number of SC product approvals per year (Figue 2).4 The benefits of SC delivery flow through to the clinic, thanks to pharmacy efficiencies with faster drug prep time, less set-up which reduces nursing time and fixed dosing which reduces waste and medication error.5-6 SC administration is a win for the patient, the clinic and the pharmaceutical company, while offering a way of differentiating the product to gain (or, in the case of biosimilar threat, slowing the loss of) market share.
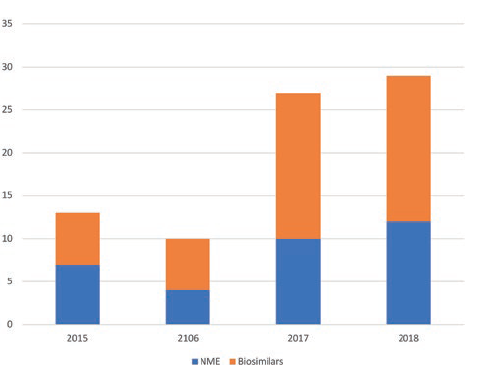
Figure 2: Global SC biologic approvals by year.
DELIVERY DEVICES AS PART OF A COMBINATION PRODUCT
Combining an active pharmaceutical product with a novel SC delivery device makes joining the world of combination devices a well-timed move. Precedents have been set by Amgen with the launch of Onpro®, an on-body injector presentation of Neulasta® (pegfilgrastim) for neutropenia during chemotherapy, as well as Pushtronex® single-use on-body infuser containing Repatha® (evolocumab) for hyperlipidaemia.
Amgen’s on-body infuser incorporates West Pharmaceutical Services’ wearable drug delivery platform – the first generation of which was the first of its kind to be US FDA approved in combination with an approved drug. These combination products have revolutionised the way a patient visualises their illness as they enable the patient to home administer their treatment, thereby avoiding a repeat trip to the hospital or clinic.
FROM DEVICE TO PLATFORM
In the drive to formulate biologics for patient adherence, higher volumes and higher viscosities are typical product profiles of many SC drugs. However, higher viscosities may not allow for conventional delivery due to the need for longer injection times to reduce patient discomfort. West recognised this trend andresponded by developing its SmartDose® drug delivery platform, which includes a first-generation device that allows up to 3.5 mL of liquid drug to be administered over a longer period.
First, human trials were conducted between 2011 and 2014, and development on wider-platform offerings – including large volume and preloaded options – started soon after. With Amgen’s FDA approval in 2016, EMA approval in 2017 and Japan/rest of the world approval in 2018, global acceptance of combination products has begun.
The SmartDose® platform has expanded with a user-loaded second-generation up to 10 mL device which leverages the success of the first-generation device with proven engineering and industrialisation on a larger scale. With options available for dose volumes up to 10 mL, the second-generation SmartDose® device can adapt to a variety of drug delivery needs (Figure 3).
“Patients have never before
been on the receiving end
of such a rapid wave of
advanced therapies.”
REASSURANCE OF KNOWING DEVICE IS PATIENT FRIENDLY
In the words of poet Alexander Pope in 1711, “to err is human”7 and this is still very apt in 2019 when we think about human factors and user error. The simplest of devices in the hands of device engineers can become a behemoth in the hands of a patient – hence the importance of human factors in Phase III trials.
Extensive human factor studies and user interface development have been done by West using the 10 mL user-loaded secondgeneration SmartDose® device. This work included body mass index (BMI), age and previous injection experience – with people who were patients (and took injections) and healthy volunteers (who did not take regular injections). The design usability, acceptability and comfort were assessed, along with establishing whether the user needs were addressed and what the monthly dosing preference was.
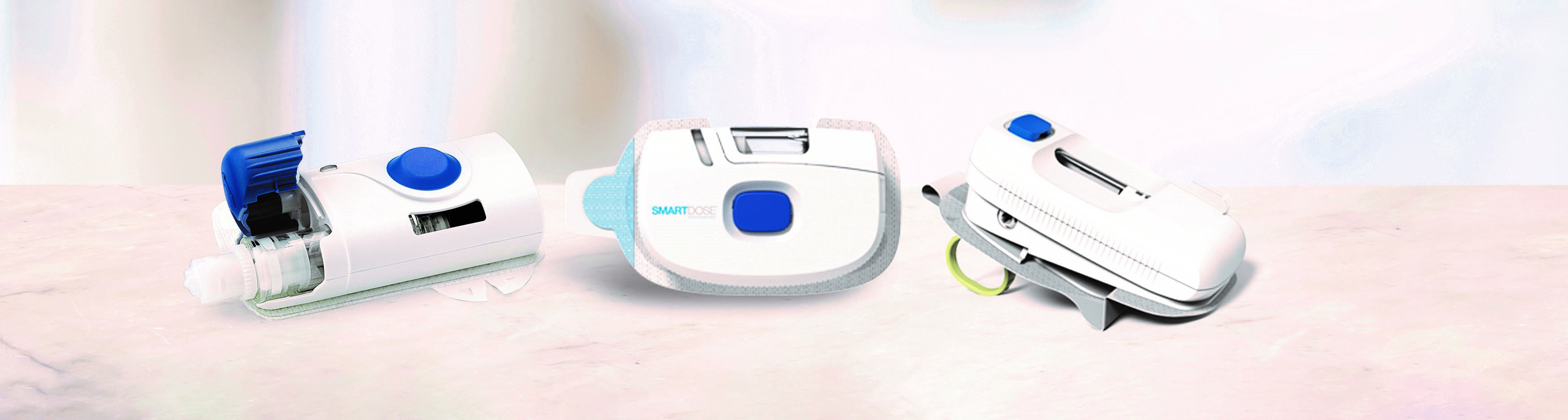
Figure 3: West’s on-body injector platform, SmartDose®.
The results showed that the secondgeneration SmartDose® 10 mL device had a well-received on-body size and was intuitive, easy to use and desirable.
When treating a chronic condition with the need for an 8 mL monthly dose, seven total treatment options were evaluated. The second-generation SmartDose® 10 mL device was voted the most preferable treatment, compared with the other treatment options, which included weekly autoinjectors, monthly dosing via multiple autoinjectors, visiting a clinic or via infusion. Patients don’t like taking frequent injections and a secondgeneration SmartDose® 10 mL device monthly injection helped them with less frequent injections.
CUSTOMERS CHOOSE SMARTDOSE® DRUG DELIVERY SYSTEM
In January 2019, scPharmaceuticals (Burlington, MA, US) announced it had signed a development agreement with West Pharmaceutical Services to incorporate its SmartDose® drug delivery system for delivery of FUROSCIX® (furosemide) – scPharmaceuticals’ lead programme for the treatment of oedema in patients with heart failure. scPharmaceuticals selected the SmartDose® drug delivery system for FUROSCIX® based on, in part, the features and functionality it offers for improving the overall patient experience.
During its recent investor day, Alexion Pharmaceuticals (New Haven, CT, US) announced that it had chosen the SmartDose® drug delivery platform for its clinical trial programme for the delivery of ULTOMIRIS® (ravulizumab-cwvz) once-weekly SC injections. West and Alexion have signed a development agreement for the first-generation SmartDose® and potentially for second-generation development for exclusive use to deliver Alexion’s ULTOMIRIS® clinical development programme. ULTOMIRIS® is used in the treatment of paroxysmal nocturnal haemoglobinuria (PNH) – a chronic and debilitating, potentially life-threatening ultra-rare blood disorder. Using the SmartDose® drug delivery platform, the drug has the potential to be the first-to-market SC option for PNH and atypical haemolytic uraemic syndrome.
SMARTDOSE® MAKES DEVELOPMENT EASIER
By partnering with West for an Integrated Solutions Program that includes regulatory support and clinical filling of SmartDose® device cartridges, customers can ease their path to market for combination products. In 2019, West announced it had commenced discussions with Swissfillon (Visp, Switzerland) – a provider of aseptic fill-and-finish services to pharmaceutical and biotechnology companies – that are intended to lead to a non-exclusive global collaboration to provide fill-finish capabilities to customers using the SmartDose® platform for complex molecules.
Through the collaboration, it is anticipated that West will be able to deliver an integrated solution with filled Daikyo Crystal Zenith® cartridges for the SmartDose® wearable device, which is expected to accelerate clinical development and enable customers to bring their innovative injectable drugs to market quickly. This new collaboration is expected to offer customers a robust fill-finish capability later this year.
AIMING FOR SUCCESS
Patients have never before been on the receiving end of such a rapid wave of advanced therapies – and biologics are at the heart of this wave. However, the ever-increasing list of demands a biologic drug places on drug developers is a significant challenge. In a price-sensitive, patient-centric world, value has never been more necessary. Working with a partner, such as West, which has proof of performance and a collaborative spirit can help navigate the challenges found along the drug development road. Let us help you to improve your patients’ outcomes, avoid costly delays to launch and provide a better return on investment. By choosing the SmartDose® drug delivery system, you choose to improve the treatment experience of your patients as they confront their illness.
Crystal Zenith® is a registered trademark of Daikyo Seiko, Ltd. Daikyo Crystal Zenith® technology is licensed from Daikyo Seiko, Ltd. Onpro® and Pushtronex® are registered trademarks of Amgen, Inc. FUROSCIX® is a registered trademark of scPharmaceuticals, Inc. ULTOMIRIS® is a registered trademark of Alexion Pharmaceuticals, Inc.
REFERENCES
- PharmaCircle, January 2019.
- Schweighofer C and Wendtner C, “First-line treatment of chronic lymphocytic leukemia: role of alemtuzumab”. Onco Targets Ther, 2010, Vol 3, pp 53-67.
- Launay-Vacher V, “An appraisal of subcutaneous trastuzumab: a new formulation meeting clinical needs”. Cancer Chemother Pharmacol, 2013, Vol 72, pp 1361-1367.
- “Novel Drug Approvals for 2018”. FDA Report, September 2018.
- De Cock E et al, “A time and motion study of subcutaneous versus intravenous trastuzumab in patients with HER2-positive early breast cancer”. Cancer Medicine, 2016, Vol 5(3), pp 389-387.
- Hendrikx J et al, “Fixed Dosing of Monoclonal Antibodies in Oncology”. The Oncologist, 2017, Vol 22(1), pp 1212-1221.
- Pope A, “An Essay on Criticism”. 1711, Part II.

