To Issue 156
Citation: Khanolkar A, White S, “An Enabling Autoinjector Technology for Delivery of Long-Acting Injectables”. ONdrugDelivery, Issue 156 (Jan 2024), pp 34–39.
Asmita Khanolkar and Susanna White explain why SMC Ltd‘s Vita autoinjector with resuspension technology provides a potential solution for the delivery challenges of long-acting injectables.
Long-acting injectables (LAIs) provide patients with a higher compliance solution for chronic diseases. However, the current parenteral formulations for LAIs are pushing the limits of traditional drug delivery device technologies. LAIs typically have high molecular weight carriers that increase viscosity and can also lead to non-Newtonian behaviour and increased sensitivity to environmental conditions. Additionally, suspension formulations can settle out during storage. Some LAIs are presented as an “in-situ forming depot” (ISFD) where the bolus shape and size can impact the controlled release and pharmacokinetics (PK). Consistent dose delivery and consistent PK are essential for a successful therapeutic outcome.
“An enabling autoinjector technology can help address the delivery challenges presented by LAIs for successful outcomes.”
Given these technically challenging delivery needs for LAIs, and the drive towards at-home administration, it is evident that there is a need for an enabling drug delivery technology platform that can be customised to the patient and technical needs of the application. The Vita autoinjector with resuspension technology for delivering intramuscular injections provides a potential solution for these types of challenging applications.
LAI FORMULATIONS
Delivery of LAIs faces multiple challenges (Table 1). Understanding the formulation is key to optimisation of filling, storage and delivery. LAIs are formulated in a delivery matrix to provide extended release over time. Depending on the formulation technology used – such as oil solutions, water insoluble suspensions or crystalline polymeric barriers – the formulations can exhibit high viscosity, non-Newtonian behaviour or phase separation in storage. This, in turn, can cause inconsistencies in delivery.
| High Viscosity | Non-Newtonian | Suspension | Stability |
|
• LAIs are formulated in a delivery matrix to provide extended release over time • Delivery matrix for such formulations typically involve high molecular weight vehicles • Increased viscosity and sensitivity to environmental conditions • Where an injected drug forms a bolus, size and shape can affect the release |
• Non-Newtonian behavior can be either shear-thickening or shear-thinning in nature • Shear-thinning (pseudoplastic) formulations pose the biggest challenge for injection • They heighten any mechanical variation in the device, which subsequently effects delivery consistency |
• Two phase systems • Delivery can be complicated due to characteristics such as phase separation, particle separation, settlement, particle agglomeration and needle clogging • Requirement for high-pressure systems to overcome these challenges • Onus on device to manage and optimise delivery |
• Compatibility with container materials • Moisture loss • Oxygen ingress • Effect of temperature, age, batch-to-batch consistency • Settling can be exacerbated by storage time, temperature and transit vibration • Some suspensions can experience particle size change during storage |
Table 1: LAI delivery challenges.
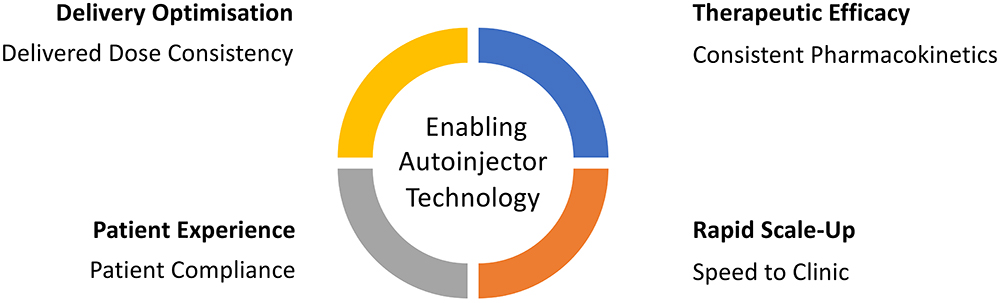
Figure 1: Key drivers for an enabling autoinjector technology.
NEED FOR AN ENABLING DELIVERY DEVICE TECHNOLOGY
An enabling autoinjector technology can help address the delivery challenges presented by LAIs for successful outcomes. Delivery optimisation, therapeutic efficacy, patient experience and rapid scale-up are some of the key considerations when developing an autoinjector solution (Figure 1):
- Consistency of Delivered Dose: Where the LAI drug is a suspension that can “settle out” during storage, an autoinjector can be designed to help “resuspend” the formulation during delivery:
• This can increase dose accuracy and homogeneity of the formulation.
• It can be particularly valuable when a suspension is opaque, such that it is difficult for the user to judge whether the formulation has been fully resuspended by shaking.
• Formulations in prefilled syringes and autoinjectors can be harder to mix by shaking than those in vials because there is a smaller amount of gas alongside the drug in the primary container.
• Shaking can cause foaming or result in a reduced therapeutic effect if any air bubble in the formulation is small. - Consistency of PK Profile: Where a drug forms a slow-release ISFD in a patient’s body tissues, a more consistent bolus shape can be formed by an autoinjector than by manual delivery. This matters if the rate of drug release is proportional to the surface area of the bolus:
• An autoinjector can provide a consistent PK profile by consistent mixing of the solid and liquid phases of the formulation.
• Non-Newtonian formulations can suffer from variable “jetting” effects that affect bolus size and shape.
• Optimisation of delivery speed can help in overcoming some of these effects. - Patient Experience: Smaller needle sizes can support compliance and prescribing preference:
• When delivering suspensions, smaller needles can be more susceptible to clogging.
• Autoinjectors can provide more force to clear clogs than a human hand.
• High-pressure container closure system is required to be able to manage smaller needle sizes.
• Needle depth optimisation for specific patient groups, considering their BMI and physiology differences. - Rapid Scale-up:
• Integrated iterative studies are required throughout the development cycle to optimise LAI delivery, adjusting formulation parameters to optimise release and PK performance.
• Test method development and implementation is also an integral part of development for LAIs to characterise the formulation’s behaviour in delivery.
• Early studies with an autoinjector solution for early-stage development are required for optimising delivery.
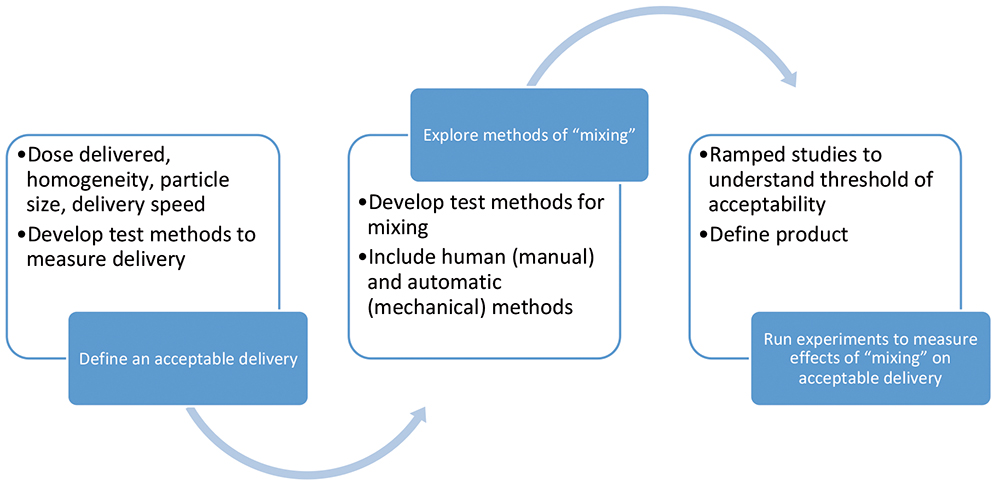
Figure 2: How to approach LAI delivery.
APPROACH TO LAI DELIVERY
“The approach to optimising LAI delivery involves focus on three areas: dose preparation, dose delivery and slow-release optimisation.”
SMC has developed some specific mechanisms to aid in the understanding of the nuances of an LAI formulation via a range of analyses, as well as potentially eliminate development challenges through careful design to facilitate resuspension of a formulation during autoinjector delivery. The approach to optimising LAI delivery involves focus on three areas: dose preparation, dose delivery and slow-release optimisation (Figure 2).
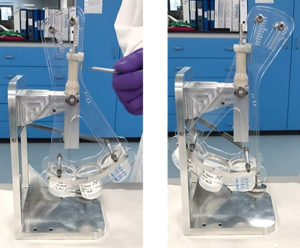
Figure 3: Segmented delivery with the evacuation rig showing (A) start and (B) end of delivery.
Dose Preparation
The first step involves defining what constitutes a good delivery. Delivered dose, delivery rate and particle size can be used as parameters to specify good delivery. The acceptable dose variability through delivery can be defined as the total dose delivered or as maximum and minimum concentrations.
Simultaneously, a worst case “settled” state specification can be defined for the formulation based on observations of stability samples, accelerated ageing and vibration testing. Samples for worst case “settled” states can be prepared through centrifuging, vibration and temperature exposure.
To investigate the concentration of drug at different stages of delivery via an autoinjector, SMC has developed an evacuation rig for segmented delivery. With a three-beaker set-up, the dose can be divided into three equal portions, as shown in Figure 3. The beakers can be weighed pre- and post-fill to ascertain weight. Using methods such as high- performance liquid chromatography (HPLC), the API content and concentration can be measured during the different stages of delivery to make sure the overall concentration meets the specification. Particle size analysis and directional light imaging techniques can be used to understand if the separation is within the acceptable level. Different formulations and batches can then be tested to understand the level of separation.
Dose Delivery
Suspensions are vulnerable to settling during storage. Unless this is addressed (e.g. through device agitation as an additional user step), it will result in a two–phase suspension with two distinct viscosities and a two-stage delivery. At the start of delivery, the plunger moves slowly as it delivers the settled sediment. However, beyond this, delivery of the remaining fluid takes place much more quickly. To combat this, an increase in the overall amount of force being used in the device allows the whole system to become less sensitive to this variation in viscosity. This approach is only possible if the container closure system can withstand the pressures required for such delivery.
SMC’s injection characterisation system (ICS) tests the flow rate of a fluid through a known needle size. A spring is placed behind the syringe plunger and used to expel the formulation while three sensors record the spring load, plunger position and container pressure throughout injection. This data is then analysed to understand the viscosity and flow characteristics of the fluid. Figure 4 shows an example of a representative two-stage delivery flow behaviour of a suspension.
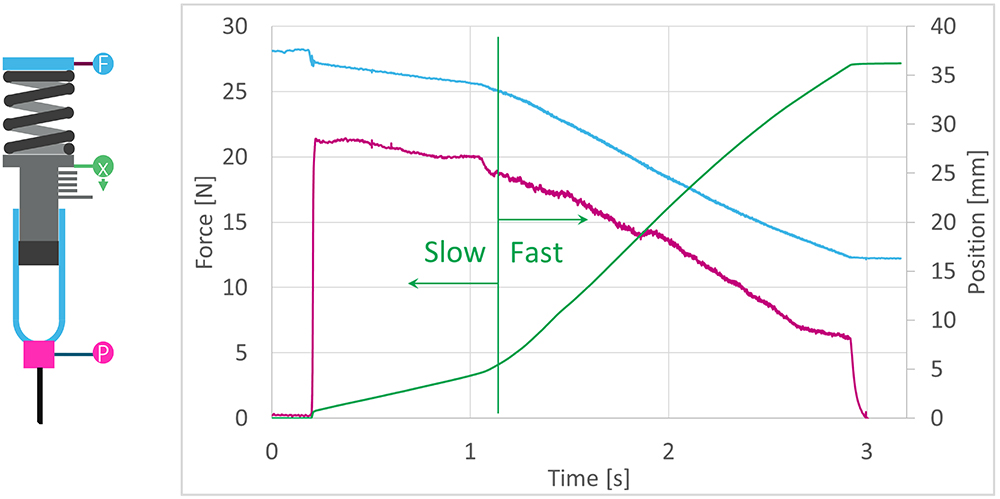
Figure 4: Optimising delivery for a suspension formulation.
Using the ICS, the delivery performance and resuspension of a “settled” formulation can be tested for delivery performance in “mixed” and “settled” states across a range of parameters. The parameters can include temperature, delivery rate, shear, needle bore and clogging effects. A mathematical model can be generated from the empirical data to enable prediction of formulation performance. SMC has developed a “mix on delivery” technology for resuspension and its effects can be studied using both SMC’s “mix on delivery” technology and user interactions.

Figure 5: CT scan for bolus shape and size.
Slow-Release Optimisation
LAI formulations are all unique and will behave differently when injected, so it is important to assess bolus formation for each individual formulation. The bolus size and shape may be affected significantly by the surrounding tissue. High-speed injections have been seen to cause “jetting”, effectively elongating the bolus, and creating a higher surface-to-volume ratio. To capture and assess the physical shape of the bolus, in vitro 3D scanning is necessary – as an example, a CT scan image of an intramuscular injection is shown in Figure 5. Where appropriate, the shape, size and position can then be quantified and compared across a range of injection parameters (needle gauge, delivery time, etc).
“LAI formulations are all unique and will behave differently when injected, so it is important to assess bolus formation for each individual formulation.”
It is also important to carry out in vivo studies to fully understand the effect that the delivery system has on drug release and, therefore, the PK profile. To facilitate these studies, SMC has designed an in vivo injection rig – an autoinjector simulator (AIS). This is a modular system that allows injections using off-the-shelf syringes in combination with a range of springs and needles to replicate the final autoinjector. As well as imitating the final delivery system, this rig is instrumented such that it is possible to acquire data on the injection force and speed of delivery for each injection. This allows a direct correlation to be drawn between PK and the injection characteristics of each individual injection.

Figure 6: The Vita autoinjector.
VITA DEVICE
The Vita device (Figure 6) is a powerful autoinjector for deep intramuscular delivery that offers repeatable and reliable performance in an intuitive, patient-friendly design that is suitable for both chronic and crisis applications.
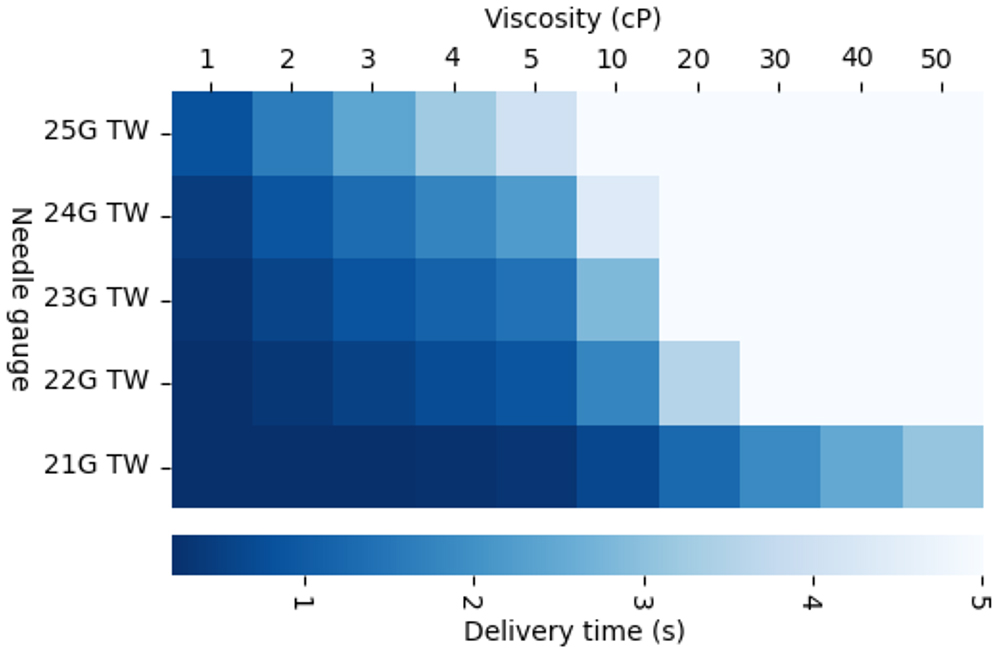
Figure 7: Vita performance.
“The Vita device is a powerful autoinjector for deep intramuscular delivery that offers repeatable and reliable performance in an intuitive, patient-friendly design that is suitable for both chronic and crisis applications.”
The Vita device can handle volumes up to 2 mL with viscosities of up to 100 cP and a variety of needle sizes ranging from 21G–25G with an exposed length of between 12.5 mm and 40 mm, depending on the patient population (Figure 7). The device incorporates a proprietary mechanism that facilitates consistent delivery of non-Newtonian formulations or drugs held in suspension, which may pose a risk of separation over shelf life. The device also features a large viewing window with visual delivery indication and both a start and end-of-dose audible indication (Figure 8).
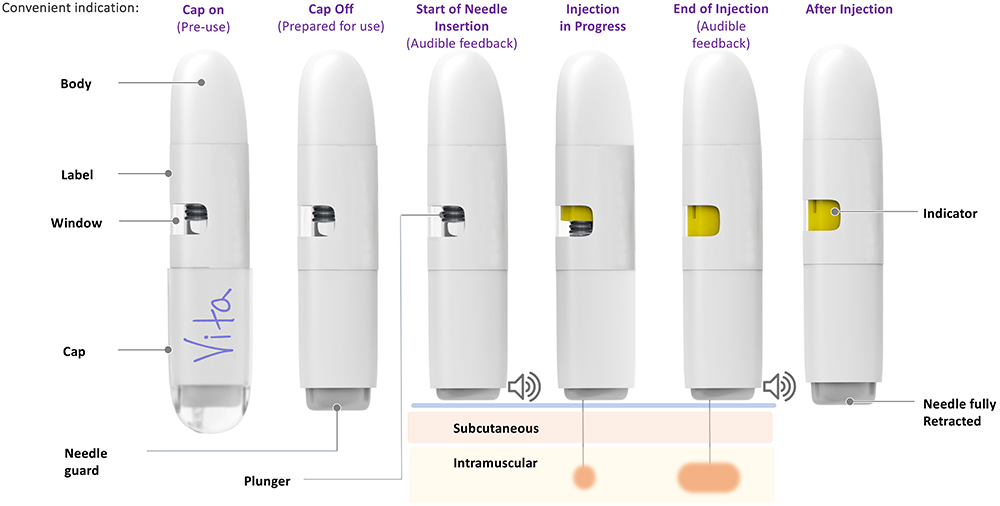
Figure 8: Vita’s sequence of injection.
In summary, the Vita autoinjector platform can offer some solutions to the delivery challenges of LAIs. With a high-pressure container system, resuspension mechanism and patient-centric design , the Vita’s features provide a unique offering. In addition, specific in vitro and in vivo mechanisms have been developed that can aid in understanding and characterising the formulation in terms of dose preparation, dose delivery and slow-release optimisation. These include the segmented delivery evacuation rig, the ICS and the AIS. Coupled with other techniques, such as HPLC, particle size analysis and 3D imaging techniques, a holistic approach fosters a greater understanding of the formulation and delivery needs in applications involving LAIs.
REFERENCE
- Khanolkar A, White S, Margerison E, “A Novel Method to Optimize Drug Delivery for Parenteral Products Involving New Therapies and Unmet Needs”. Pharm Res, 2023, Vol 40(10), pp 2303–2315.

