Citation: “Interview: Benjamin Dietiker, Weidmann Medical Technology”. ONdrugDelivery, Issue 117 (Mar 2021), pp 18–21.
Benjamin Dietiker is Key Account Manager, Pharma Primary Packaging at Weidmann Medical Technology, a Weidmann Group company and a leading developer and producer of innovative high-quality injection-moulded plastic components. He is responsible for key accounts in the pharmaceutical market, for business development and strategic innovation projects. Mr Dietiker has a BSc in Engineering & Management from the FFHS University of Applied Science in Switzerland.
In this exclusive interview with ONdrugDelivery, Mr Dietiker discusses how the company has successfully implemented a growth strategy over the past six to seven years by focusing on key areas, plans for expansion, the effect covid-19 has had on the business, and how the company differentiates itself from larger competitors by focusing on quality and service.
Q What changes have occurred in the industry over the 25 years that Weidmann Medical Technology has been providing injection moulded plastic components, and modular assembly and packaging solutions, in particular with regard to prefillable syringes and other parenteral primary packaging such as wearable injectors?
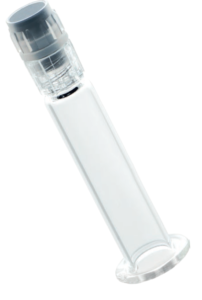
Figure 1: Customised syringe barrel with threaded sealing closure, comprising tip cap, rigid cap and Luer lock adapter. Components are steam sterilisable, medical grade, and biocompatible per ISO 10993-1 and USP Class VI.
A From our perspective as a producer, some of the most marked changes we’ve seen in terms of the requirements from our customers who are active in the field of combination products relate to increasingly stringent regulatory standards around the detection and monitoring of particles, microbiology, and in-line testing. We take such customers’ demands very seriously and we can adapt rapidly. For instance, we are currently able to produce prefilled syringe closures in an ISO class 5 environment, whereas ISO class 7 or 8 requirements are the usual industry standard.
From a material perspective, the majority of the world’s prefillable syringes are still produced with glass barrels, but in recent years we have seen a notable increase in the proportion with plastic barrels – such as prefilled syringe barrel with threaded closures (Figure 1). There is an industry trend towards plastic and also hybrid (glass/polymer) materials.
Over the past decades, there has also been a move away from parenteral medication always being administered in the clinical setting towards self-administration at home.
Q Please can you expand on the move towards self-administration?
A Moving from clinical point-of-care towards self-administration at home can ease the burden on hospitals and doctors, but it can also help to make treatments more convenient for patients, which ultimately helps to reduce the healthcare costs and improve outcomes. So pharmaceutical products and the devices used to deliver them have evolved and adapted to this trend, becoming safer, more intuitive and suitable for use by patients. Likewise, devices have evolved in response to the rise of biologic-based therapeutics. For example, more viscous formulations and higher dose volumes have become more common and so devices with larger capacities – including prefillable syringes with larger barrels, and wearable injectors – have emerged.
Q What changes have occurred in the industry in terms of technology?
A One very significant change has been the emergence of digital technologies, in particular the Internet of Things (IoT). On the patient side, we have seen the rise of connected devices. Today, diabetes patients can monitor their glucose level through a wearable continuous glucose monitoring (CGM) system and administer the requested insulin amount through a wearable insulin pump. This is a great example of how technology can increase patient quality of life significantly.
I am very proud to be part of an organisation that rises to the challenge and has the know-how and capabilities to co-develop and produce such high-quality devices, maintaining product quality without compromise. For example, we offer repeatable high precision production under the highest clean room environment standards. We worked hard to acquire the specific skills and knowledge required to achieve current quality levels and as industry and regulatory expectations grows, likewise we are always striving to improve still further with every day.
“Any customer can see at any given time where their production stands; if they’re fully loaded, what their OEE would be, and many other valuable metrics.”
Q Can you talk about how the industry is seeking to improve product identification and traceability?
A That is indeed highly demanded, not only by our customers in the prefilled syringe field, but also by regulatory health authorities. The US FDA describes under 21 CFR section 610.14 the identity requirements for container products. There are increased requests not only to mark products, but to include information about the material and batch number directly on the container for biologics. Colour coded rings are the identification method of choice today, but there are some constraints regarding investments and requested floor space for labelling lines, limited flexibility and data readability in assemblies, to name a few.
Q What can Weidmann Medical Technology contribute to meet that demand?
A We started to look at the integration of the radio frequency identification (RFID) technology into plastic products through injection moulding techniques more than 10 years ago. I would say we were pioneers at that time, and probably even a bit early, but we gained knowledge and kept improving on our methods. Today, our previous investments seem to have paid off and we believe we bring important value to the table here. We can support our customers straight away with our knowledge and our RFID tagging and labelling as well as an RFID reader supplier base. And, most importantly, we are very lucky to have a team of dedicated and experienced development engineers, who are able to accommodate requested RFID tag or label designs, together with our suppliers, to fulfil our customers’ needs.
Q Have you also implemented connectivity within your manufacturing facilities?
A Absolutely. On the industrial side, the way our factory and machines are connected has certainly completely changed. Today we run a state-of-the-art MES (manufacturing execution system) and CAQ (computer-aided quality). The cloud-based MES software supports all tasks connected with planning and controlling equipment and production processes. It allows real-time monitoring (Industry 4.0), meaning that any customer can see at any given time where their production stands; if they’re fully loaded, what their OEE (overall equipment effectiveness) would be, and many other valuable metrics. We use it for preventive maintenance and continuous improvement processes (CIP) with a target to cut cost for our customers. Some customers just harvest that data and keep it as peace of mind, others really put this data to work for their own purposes. It’s an area where we truly differentiate and add value. The offering around our MES is a prime example of one key way in which we’re differentiated from our competitors. When it comes to transparency, we are ready and able to share this kind of real-time data with our customers.
Q Please could you describe some of the changes that Weidmann Medical Technology has undergone over the past few years, and the growth that the changes have prompted?
A We certainly noticed that the industry has become far more competitive and cost-conscious over time, and so has Weidmann Medical Technology. We only expect to receive projects if we offer both excellent quality and value. The Weidmann Group has been in existence for more than 140 years, it is family owned, with a reputation built on Swiss quality. In the past, Weidmann Medical Technology was very R&D driven, and a bit of a “jack of all trades” in that we catered to five markets, including labware and, most dominantly, microfluidic applications. We’re still using the knowledge that we established during our stint in microfluidics for present production. But we found the R&D-driven strategy made it difficult to generate significant growth so, in 2014/2015, we refocused on production, and we streamlined our portfolio tremendously to cater to just two or three markets: pharma (predominantly primary packaging closures); IVD (in vitro diagnostics); and also medical devices.
“The feedback we get from customers is that we are always available to them.”
Q What has changed since you streamlined your portfolio and refocused on production?
A Since refocusing, we’ve experienced steep growth in these key areas, and we remain aware that as a medium-sized company we always have to maintain that very tight balance between R&D and growth. When we do R&D, it has to be bespoke R&D directly related to a customer’s demand or project. As mentioned, we have developed specific know-how in the field of RFID in the field of prefilled syringes, which can be seen as one of our USPs (unique selling propositions) and that helps us to secure our position in the market. In line with our strategy, this is not just a research concept. Rather it comes from collaborating (co-development) with our customers on a live project, on how to implement RFID technology into their existing products. We’ve helped them develop the right design and manufacturing approach, the geometry for the RFID tags and finding the right suppliers. We can confidently say that we have established deep experience in over-moulding of RFID tags or chips in existing products. But for us the most important thing is that we focus on production, keep all our processes state-of-the-art, develop them and bring additional value our customer.
Q How has covid-19 affected the company?
A Having been focusing on the IVD market for several years, as the covid-19 pandemic emerged we were in the fortunate situation that we could provide the market, namely PCR and point-of-care testing consumables, with new and existing products, and two of the world’s market leading companies in the IVD area were already longstanding customers. As a result, we have seen substantial growth in 2020 and this will continue in 2021.
We received significant orders not only for existing machines, but also for new production lines, which have been implemented in record time. It was an impressive experience working with such major companies at such lighting speed. This was done without cutting corners of course, but the pandemic made it necessary for projects to be accelerated. That was done on both sides very, very successfully. Everybody was pulling together in the same direction – our suppliers, sub-suppliers, customers and ourselves alike. It fills us with pride, to be honest, because our products go directly towards the covid-19 testing effort.
Q Is this success only related to your Swiss site in Europe?
A No, it’s not. We were fortunate to be able to take the momentum and expand our Mexican site, which is very exciting for our organisation and our colleagues overseas. Our Mexican operators are already receiving comprehensive training at our site in Switzerland, and the tech-transfer of the entire injection moulding machinery, tools and assembly lines is expected to happen during the coming months. Our long-term trusted partnership with one of the world’s major IVD players has made this possible. This move will certainly strengthen our position in the North American market.
Q How did the pandemic affect your organisation operationally?
A The pandemic did affect us operationally, as it did all organisations, and we took all the appropriate safety measures of course.

Figure 2: To keep its team safe during the pandemic, Weidmann Medical Technology divided operations by cleanroom rather than by shift.
At a very early stage, the Swiss government granted the Weidmann Group divisions the status of being system relevant to essential infrastructure. In order to achieve the rapid project turnarounds the pandemic demanded, whilst at the same time keeping our team safe, simply put we divided our operations by cleanroom and no longer by shift (Figure 2). We have more clean rooms than we have shifts, and so the advantage of this system is that if there had been somebody infected with covid-19 we would not have had to send the whole shift home as a consequence, but only the team of one cleanroom. So far, fingers-crossed, we have successfully isolated the few cases that we’ve had, and we have avoided any co-worker at our facility contracting the disease from somebody onsite, so I would call that a success.
Q Do you see the recent growth being sustained, and will it feed through to an uptick in demand on the delivery systems and parenteral packaging side of things as well as IVD?
A I would express our firm assumption that this is not a short-term trend. It’s a long-term change that started before the pandemic and will persist after covid-19. The perceptions of people, the perceptions of companies and the perceptions of governments will have all changed. We will find it much more important to test more frequently and to test more widely even when we have beaten the pandemic. Obviously, it starts with IVD but demand for primary packaging will follow because treatments and vaccines against the disease need to be administered.
Q What are the main insights you’ve picked up from trends in enquiries from customers?

Figure 3: A needle shield incorporating a specially developed thermoplastic elastomer (TPE), mass produced with highly complex multi-cavity moulds.
A In one sense, because we are already producing, for example, needle shields (Figure 3) and prefilled syringe closures, enquiries come through because the industry community knows already that we are doing this. But then, as mentioned, during the pandemic, enquiries relating to covid-19 testing consumables have surged for obvious reasons and, with regards to prefilled syringes, we’ve been asked about prefilled syringe needle shields, soft and rigid closures, threaded closures as well. Enquiries have not been limited to closures but have also been about polymer syringes. Lately, potential customers have been asking about the capacity we have, what our capacities are in terms of cleanroom space (Figure 4). We have one Swiss production site and, as we have touched upon, one in Mexico, and we’re underway establishing a third production site, also in Switzerland, at our headquarters.

Figure 4: Weidmann Medical Technology has a Swiss production site, another in Mexico and is establishing a third large production site at its Swiss headquarters.
Q Tell us more about your new site at your headquarters in Switzerland.
A This is most exciting news. Because of the solid growth of Weidmann Medical Technology over the past few years, we decided to expand our production capacity at our headquarters in Rapperswil, Switzerland. The beauty is that the expansion will take place in an existing Weidmann Group facility, previously used by Weidmann Electrical Technology, in the very centre of Rapperswil. This is a substantial milestone for the entire Weidmann Group and a clear commitment to the Swiss location. It is a site with substantial capacity because we have the demand for large machines and production lines. This expansion is not related to covid-19; the site was established more than a year ago and we have begun implementing the lines. The overall potential is huge here at our headquarters. By 2025 we expect our production site to be >6,000 m2.
Q Weidmann Medical Technology prides itself on a truly differentiated service offering to its customers, unparalleled transparency and dedicated people for each customer. Could you go into a little bit more detail about this service offering?
A Weidmann Medical Technology has explicitly only ever grown organically and growing organically just takes a longer time. That’s a rule of nature. Weidmann Group itself has been around for more than 140 years, employs almost 3,000 people, and generates a turnover of around CHF350 million (£270 million), so we benefit from being part of that large group. But still the medical division, in comparison with our competitors, is rather small and so we need to find out where it can make a difference. For us that is certainly customer centricity. It’s part of our DNA, we are family owned. I would say that we can turn around projects much faster than our bigger competitors because we can make decisions and release budgets much faster. This has been of value for customers, we have that feedback. Additionally, although it’s not necessarily a focus, we are willing to accept smaller orders, let’s say, one line, a second-source project, or maybe an end-of-life project. The experience we have had is that being willing to help a customer out on these sorts of projects, which we are allowed to do by our management, builds up trust and loyalty which is not forgotten when something more attractive comes around the corner. The feedback we get from customers is that we are always available to them, at least for a call, if not a visit.
Previous article
ROOM FOR INNOVATION?Next article
I-PLATFORM DEVICE: THE SMART DEVICE WITH APTITUDE
