To Issue 182
Citation: Goldfarb L, “Building Strategic Partnerships for Early-Stage Drug Delivery Device Testing”, ONdrugDelivery, Issue 182 (Jan 2026), pp 48–52.
Visit Instron at Pharmapack Paris! – Stand 4D95
Landon Goldfarb of Instron considers the importance of relationships between formulation and delivery teams for pharmaceutical innovation, highlighting a framework to differentiate between an equipment supplier and a testing partner, outlining the system capabilities and organisational competencies required to create effective early device-development teams.
The pace of pharmaceutical innovation is growing exponentially, driven by novel mechanisms of action and AI-powered drug discovery, which leads to robust drug pipelines. This pace has thrust organisational excellence in drug delivery systems into the spotlight, as many newer formulations have characteristics such as higher viscosities or lyophilised products that can challenge traditional delivery devices. This, along with consumer expectations for self-injection devices, means that drug developers need to investigate delivery devices earlier and more often, creating dedicated teams to assess the current landscape of devices to meet their existing and future pipeline.
These early-stage functional groups require testing equipment that provides both modularity for various device form factors and user experiences built around intuitive method development. Understanding the critical requirements for this equipment can allow early device teams to adapt to potential formulations with agility and evaluate a wider range of device technologies.
THE RISE OF DEVICEABILITY FUNCTIONS
The chasm between the formulation and delivery teams has begun to slowly narrow, as project success is much more closely linked to the choice and subsequent performance of the drug delivery system. To build these crosslinks, communication between these teams is happening earlier and more frequently, allowing for discussion of pipeline strategy over longer timescales.
Restructuring occurs on different scales depending on the size of the pharmaceutical organisation. For major players, this has involved expanding core competencies to develop new functional groups, while for smaller players, this can mean being more deliberate in identifying contract partners to support their device development needs. In either case, a common intention around device strategy has created both benefits and challenges for these organisations.
Holistically, this cross-functional effort can make both sides more successful. A stronger shared understanding of formulation and subsequent delivery platforms can help to identify potential issues earlier and create more effective feedback loops. As an example, biological formulations typically require cold storage, with some messenger RNA therapies requiring ultra-cold storage below -80°C. These conditions can have a major impact on device performance and container closure integrity. In an ideal scenario, deviceability teams will have performed preliminary investigations and studies to identify the performance characteristics of different platforms, providing insights into syringe barrel siliconisation, elastomeric components and break-loose and glide forces.
Such insights can circumvent time spent evaluating different devices or, more crucially, going down the wrong path. Furthermore, performing preliminary device testing can provide data that can supplement design verification for the combination product. This advanced development approach can streamline the selection process and ultimately shorten time to market.
The inherent downside is simply the novelty of these functions for some organisations. In many cases, the supply of device-oriented engineers cannot meet the demand. Particularly among smaller companies, more analytically focused talent is being moved into the device space, often using unfamiliar equipment. This disconnect highlights the importance of selecting a partner that understands the unique needs of the drug delivery space and can offer equipment designed for usability. Building a talent pool capable of developing robust device evaluation protocols is critical for ensuring that there are vetted devices ready to support current and future drug pipelines.

Figure 1: Instron Autoinjector Testing System, capable of full functionality testing for needle shield and button-activated devices, as well as safety syringes.
SELECTING THE RIGHT EQUIPMENT PARTNER
Identifying the right partner requires evaluating both their physical equipment capabilities and their organisational experience within the drug delivery space. A partner with both will be best poised to support an expanding deviceability team, with considerations towards long-term needs including design verification and transfer to production. These long-term considerations can help avoid roadblocks associated with method transfer between systems, functional groups or sites and more quickly identify potential device issues earlier in development (Figure 1).
Compatibility With Platform Devices
The use of autoinjectors has become normalised for many patient populations, spurred by the rise of glucagon-like peptide-1 and other chronic therapies. In many cases, automated drug delivery systems are an expectation from the market rather than an additional feature. This expectation has led to unprecedented growth for makers of platform devices, such as Ypsomed’s YpsoMate™ or SHL Medical’s Molly™, which have built global infrastructures designed to support production ramp-ups and have significant clinical data to de-risk their implementation. These factors have led many pharmaceutical organisations to pursue platform strategies, choosing to standardise on a platform for multiple assets. This allows for preliminary testing that can then be bridged into later phases of the development process.
Considering the risk-averse nature of the pharmaceutical industry, it is likely that platform devices with considerable time on the market will be the initial contenders for deviceability groups. Ideally, testing equipment will be automatically configured to support the most common platform devices on the market, while minimising the amount of physical and method setup required to perform device evaluations. Reducing the complexity of changeovers and the number of interchangeable parts helps to reduce equipment setup time and training required for operators. Figure 2 shows an example cap adapter, which allows for seamless transition between platform devices with a single change part. Collaboration between the two means that the test system has been specifically designed with these platform devices in mind, ensuring proper fit and function of the system.
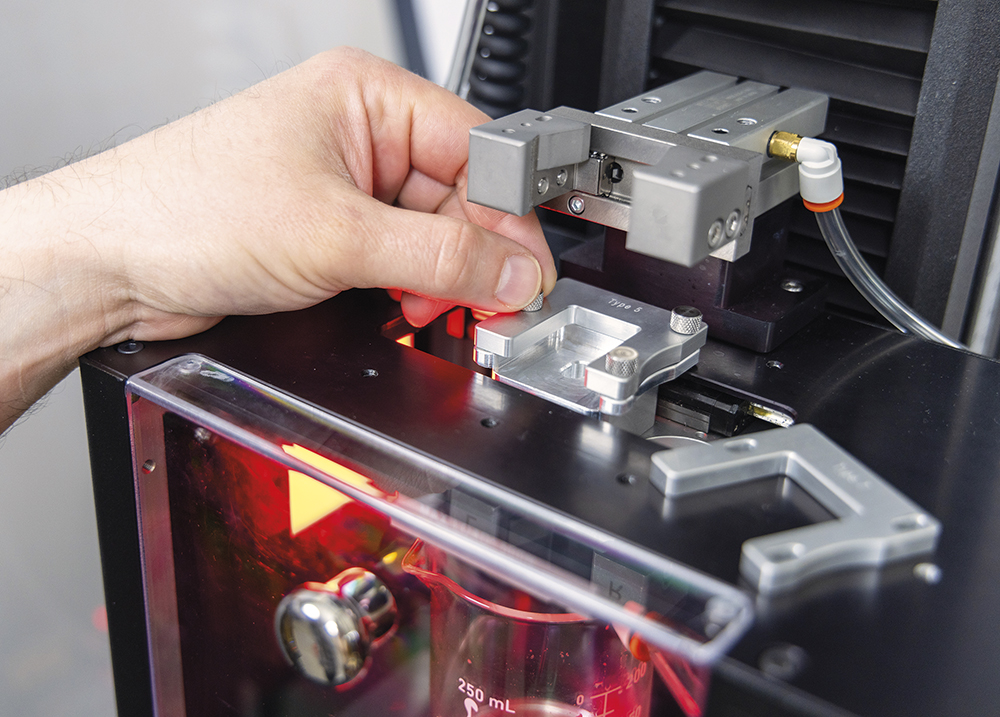
Figure 2: Example cap adapter used to switch between testing different platform devices on Instron’s Autoinjector Testing System.
Beyond physical integration, method development is another area where an expert partner can add value. Alignment of test protocols between the device manufacturer and the pharmaceutical company can help to avoid measured performance outside of the expected specification. When these issues arise, the root cause analysis can be time intensive and require significant collaboration between both parties. In many cases, the equipment supplier is expected to assist, helping to isolate variables in the test procedure that could result in a discrepancy. These issues can be mitigated if the equipment supplier has channels of communication with the device manufacturer and insight into their internal procedures. With that connection, methods can be delivered directly to the customer that align more closely with those of the manufacturer – removing problematic variables and leading to faster solutions. Additionally, this relationship can ensure that test equipment capabilities align with device manufacturers’ future product roadmap, limiting the time needed to support newly released devices.
Supporting Novel Device Requirements
Today’s drug delivery device landscape is ever growing and changing, driven by patient needs, drug pipelines and industry trends. Serving unique patient populations, paired with increased emphasis on human factors as an organisational competency, is directly impacting the functionality and form factor of devices on the market. For example, devices are using novel feedback mechanisms, shifting from individual auditory clicks to continuous clicks throughout the injection, developed after studies of patient reaction time.
“IN THIS INDUSTRY, SUSTAINABILITY IS PARAMOUNT AND, IN MANY CASES, DIRECTLY SHAPES THE DEVICE FORM FACTOR.”
New modalities being brought to market require delivery via lyophilised solutions and reconstitution, necessitating devices with additional patient interactions and capabilities. In this industry, sustainability is paramount and, in many cases, directly shapes the device form factor. An example of this is using a reusable body with interchangeable cassettes loaded with the primary container. These factors are spurring the development and commercialisation of innovative devices, each with the potential for unique testing requirements. Many of these devices are being developed by smaller manufacturers because they can be more agile in response to industry needs, or with the intention of addressing a specific use case. For this reason, many organisations are broadening the scope of devices they are investigating.
Figure 3: Modular torsion add-on enables biaxial testing capabilities.
As an example, many devices designed for lyophilised drug products require additional patient input for the reconstitution process. Unlike most platform devices, this can involve a rotation in addition to push-pull actions. This additional axis needs to be evaluated to discern the torque necessary to engage the mechanism, which adds complexity to the testing process. Test systems need to be expanded to include the measurement of torque and angular displacement in addition to the standard functional assessment. To guarantee reliable results, the system should allow for programmable rotational rates and a rotary encoder to plot the torque profile in relation to the angle. This level of modularity is crucial for enabling highly capable test programmes and should be a prerequisite for selecting a potential system manufacturer (Figure 3).
Another example has resulted from changing international standards for testing injectable drug delivery devices. As these devices become more commonplace, standards are updated accordingly to better reflect the landscape of devices on the market and their essential performance requirements. ISO 23908 outlines the requirements for sharps protection features on drug delivery devices. The most recent update in 2024 saw increased scrutiny when testing access to a device while in safe mode. For autoinjectors, this specifically refers to when the needle shield is locked out. Traditionally, this has been done through dimensional stack-up analysis and finite element analysis. Physical tests are increasingly preferred, especially when performed as a variable test. This reduces the total number of samples needed, a critical efficiency gain in early-stage programmes when devices can be scarce. Needle safe distance, or the measurement of the distance between the needle tip and the needle shield in the locked-out position, is another common modular add-on.
Mechanical test equipment should allow for modularity, supporting additional test capabilities as needed. Beyond available add-ons, an equipment partner should have in-house design teams with a competency around developing custom modifications. When using the equipment, manufacturer-provided concepts offer additional benefits – including method development support, traceability and simplified transfer to production.
“REGULATORY COMPLIANCE SHOULD BE DISCUSSED EARLY AND OFTEN WITH EQUIPMENT MANUFACTURERS, ASSESSING THE SYSTEM’S ABILITY TO BE USED IN GOOD MANUFACTURING PRACTICE ENVIRONMENTS.”
Scalability for the Future
Investing in test equipment in the early device evaluation stages does not need to be a trade-off with considerations for later-stage objectives. Regulatory compliance should be discussed early and often with equipment manufacturers, assessing the system’s ability to be used in good manufacturing practice environments. Performing daily checks on equipment is an essential capability to ensure measurement devices are operating within specification before collecting data and instilling confidence in the results produced by the system. Using system-integrated daily check devices removes the guesswork for organisations. Additionally, built-in software workflows can reduce documentation burdens, integrating the operator sign-offs and daily check reports directly into the system audit trail. Moving towards digital documentation and automatic prompting for daily checks minimises errors in data collection and ensures traceability (Figure 4).
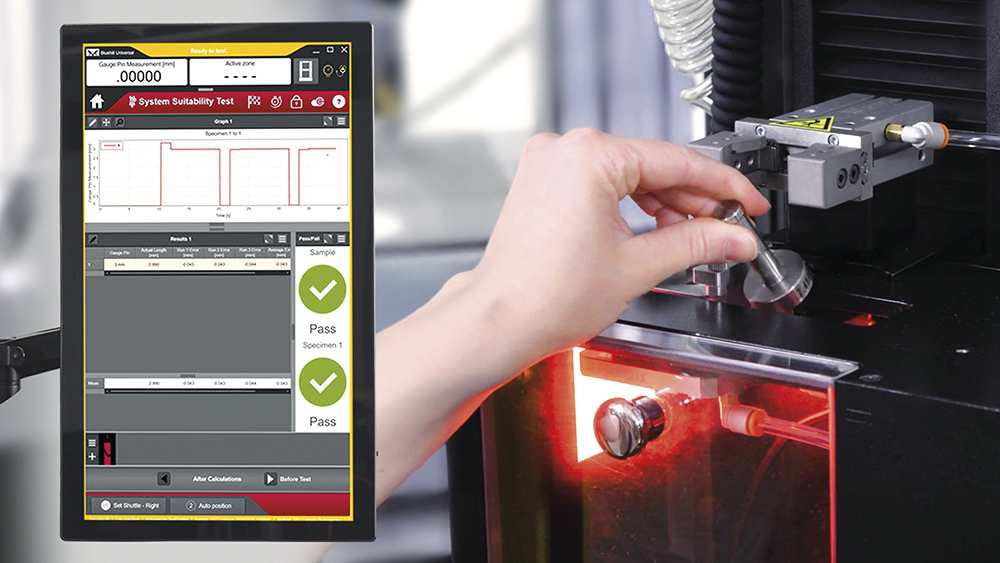
Figure 4: System suitability testing ensures measurement devices are operating within specification before collecting data.
Method development will often begin with the early device teams – with their work being the foundation for design verification – followed by production environments. Enabling change management of test methods and the ability to transfer methods across different functions, can remove uncertainty related to method selection and prevent accidental modifications to validated methods. Enterprise software platforms are scalable with pharmaceutical organisations and can connect test systems across sites – unifying method databases and user permissions, and restricting operator actions to avoid errors.
Instron’s Bluehill Central lab management software enables remote management of connected testing systems, allowing for the creation of teams, each with their own dedicated permissions and file repositories – meaning data can be siloed according to function and asset programme, then transferred when necessary. Systems capable of supporting these software platforms allow for seamless hand-offs across the development process and should be a key metric for selecting a partner.
Finally, when looking towards higher-volume, more standardised test environments, it is important to consider automation capabilities. Automation serves to reduce opportunities for operator error and variability in the data. In most autoinjector-specific cases, automation will not actually reduce the total test time, so it is important to identify automation designed to reduce requirements for system setup and device changeovers. The level of reliance on equipment manufacturers can be prohibitive, especially in work environments where employee turnover is an issue. System flexibility and usability are paramount in evaluating automation partners.
These criteria provide a framework for differentiating between an equipment supplier and a testing partner, identifying the system capabilities and organisational competencies necessary to assist in building effective early device-development teams and, subsequently, to navigate the entire development process.

