Citation: Klein M-C, Bilstein A, Kraus A, “Challenges Besides Drug Targeting – Regulatory Hurdles for Multidose Nasal Sprays”. ONdrugDelivery, Issue 154 (Nov 2023), pp 73–76.
Marie-Christine Klein, Andreas Bilstein and Rouven Kraus, discuss recent regulatory changes affecting drug-device combination products, using the example of a multidose nasal spray.
“The olfactory region can be targeted directly by adjusting multidose nasal spray devices to provide a slow-moving fine mist that has ideal properties to deposit deep within the nasal cavity.”
Targeted drug delivery is key to maximising the potential of state-of-the-art drug developments. In order to support targeted drug delivery, application devices combine a number of characteristics to ensure performance and patient safety: a precise dosage, the technical prerequisite to reach the target of interest and a good usability profile.
A well-known route of drug targeting is the nasal route, which is used by nasal spray devices that have been used by patients all over the world for decades. A prominent example of indications that make use of the nasal route is, of course, coughs and colds. But a lot of other indications also benefit from the nasal route because of its advantageous properties, such as high drug uptake because of good vascularisation and systemic circulation and the possibility of bypassing the liver.
Furthermore, the olfactory region can be targeted directly by adjusting multidose nasal spray devices to provide a slow-moving fine mist that has ideal properties to deposit deep within the nasal cavity.1 This adds the advantage of providing access to the brain by nerval structures that connect the brain and the nose – the so-called trigeminal and olfactory pathways – and of treating brain-associated disorders of the central nervous system, such as Alzheimer’s and Parkinson’s disease.
REGULATORY ASPECTS OF NASAL DRUG DELIVERY
Market-ready nasalia require the development of a formulation that fits the nasal physiology and a suitable nasal spray that can transform the formulation into a spray with defined properties that lead to an effective and safe treatment. In addition, regulatory requirements pave the road to market and are key to successful development and market authorisation.
This article will elaborate on a specific aspect of regulatory provisions that has changed significantly over the last few years and which the Regulation (EU) 2017/745 on Medical Devices, better known as the MDR, brought to the field of drug-device combinations – a medicinal product administered by a multidose nasal spray.
From a regulatory point of view, the targeted administering function of a nasal spray with its defined doses leads us to the intended purpose of the multidose nasal spray, which characterises the spray pump as a medical device. With that in mind, along with the MDR, it becomes clear that nasal sprays are very often part of drug-device combinations (DDCs). In a nutshell, DDCs contain both a medicinal product and a medical device. But, of course, different scenarios of these subsets of combinations exist and each combination requires a strong regulatory examination focusing on the definition of the principal mode of action. Depending on the latter, either the MDR or the Directive 2001/83/EC defines the regulatory landscape of the DDC. These DDCs and their intertwined regulatory provisions with the Directive 2001/83/EC are addressed in different articles of the MDR.

Figure 1: Nasal pumps are an integral part of the drug-device combination product, according to MDR.
MDR article 1 (8 and 9) provides the definitions of incorporation and administering and outlines the concept if it is an integral device. And MDR article 117 directly amends the Directive 2001/83/EC in terms of DDCs and regulatory requirements concerning the device part in MAAs. The following sections concentrate on the respective aspects that are of interest in the example of a nasal spray used to administer a medicinal product (Figure 1).
Nasal Spray Pump as an Example of an Integral DDC (MDR)
Nasal spray pumps work by transforming a liquid formulation into a spray and targeting the drug to the nasal cavity. By industrial processing and aseptic filling, they are filled with the respective (sterile) formulation and, as a consequence, are fused into a single integral product that is intended exclusively for the use in the given combination. Moreover, the nasal spray pump is not reusable. All these characteristics are the subject matter of Article 1(9) of the MDR that leads the reader to the following regulatory strategy: the DDC in that case is governed by the medicinal products framework and the device part (nasal spray pump) needs to fulfil the general safety and performance requirements (GSPRs) outlined in Annex I of the MDR.
“The MDR has changed the European medical device regulatory
landscape fundamentally.”
Nasal Sprays to Deliver Medicinal Products: MDR Article 117 Applies
The MDR has changed the European medical device regulatory landscape fundamentally. Within the MDR, there is more emphasis on post-market surveillance and clinical data, which substantiated the focus of interest on the product’s whole lifecycle (Figure 2). In the same vein, the MDR also has influence on the MAA of medicinal products. Article 117 of the MDR alters the Directive 2001/83/EC in the case of products that combine a medical device part with a medicinal product in cases where article 1 (8, 2nd sub) or article 1 (9, 2nd sub) of the MDR apply.
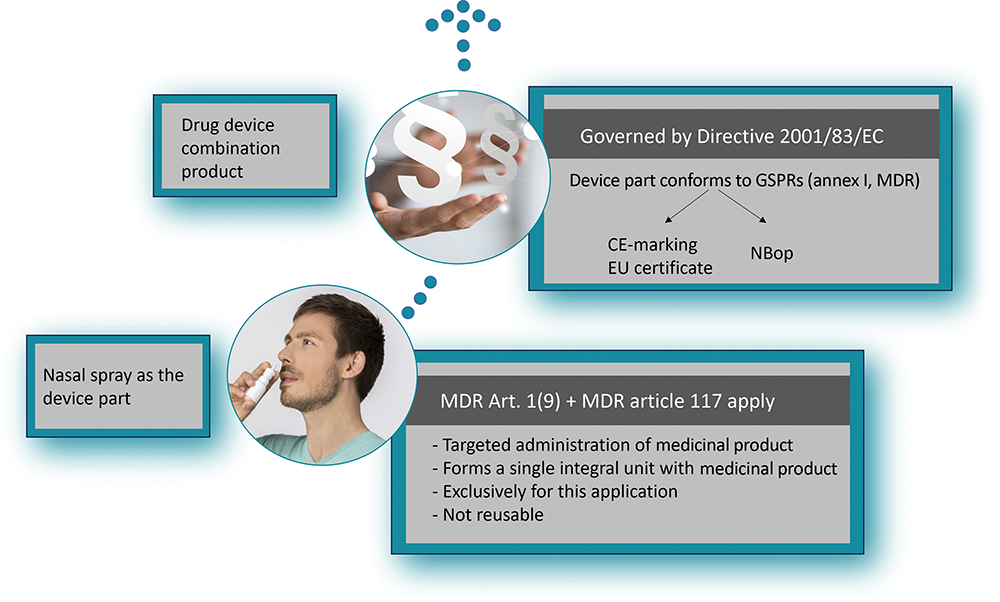
Figure 2: Regulatory route to market for DDC products, exemplarily for a medicinal product applied in a nasal spray.
“URSATEC and Aero Pump provide medical pumps CE marked with full MDR-compliant documentation that supports conformity with the GSPRs.”
Article 117 contains the requirement for the marketing authorisation dossier to contain the results of the conformity assessment of the device part with the GSPRs or the relevant certificate issued by a notified body (NB) allowing the CE-marking of the medical device. If the marketing authorisation dossier does not contain a conformity assessment although the device part itself requires the involvement of an NB, the authority will require an opinion on the conformity of the device part to the above-mentioned requirements (NBop).
MDR Influences Marketing Authorisations for Medicinal Products
The good news first: for marketing authorisations that have been granted before the due date May 26, 2021 that did not undergo significant changes, this regulatory change is not effective – it is not intended to apply retrospectively. However, for marketing authorisation holders of nasal drugs that do not yet have an unlimited market authorisation, the due date applies.
Since May 26, 2021, the effective date of the MDR, new MAAs need to fulfil article 117 of the MDR. The latter is also effective for renewal applications at the end of the five-year initial marketing authorisation. Nine months before validity is due to run out, re-evaluation needs to be applied and, when granted, provides the prerequisite for an unlimited marketing authorisation. With this application, information on all variations introduced since the marketing authorisation was granted needs to be given, which may also require the conformity assessment or the NBop of the device part of the DDC. In general, significant changes to the design and intended purpose of the product may be influenced by article 117 and require action, even if an unlimited market authorisation was already granted before due date.
The EMA has issued a Questions and Answers Document2 for applicants, marketing authorisation holders of medicinal products and notified bodies with respect to the implementation of the MDR and IVDR,2 which outlines the above-described situation in detail. Moreover, a guideline on quality documentation for medicinal products when used with a medical device is available that helps with incorporating the regulatory requirements of the MDR into the quality part of the marketing authorisation dossier.3
Medical Pumps: Class I(S) Medical Devices According to MDR
With a strong focus on the evolving needs of the changing regulatory environment in the EU and what is requested by authorities, URSATEC and Aero Pump provide medical pumps CE marked with full MDR-compliant documentation that supports conformity with the GSPRs. Furthermore, in the case of medical pumps being used in medicinal products declared sterile, a class 1s EU certificate issued by an NB is available. With this added value, customers are provided with a hands-on solution when using delivery devices for nasalia in cases where article 117 applies (Figure 3). Ideally, these regulatory prerequisites coming from the MDR, and changing the provisions for medicinal products in the case of DDCs, are implemented at a very early stage of development.

Figure 3: Customers are provided with a hands-on regulatory solution for nasalia.
REFERENCES
- Klein MC, Kraus R, Bilstein A, “Nose-to-brain drug administration and a novel approach with an old familiar”. ONdrugDelivery, Oct 2022, 131, pp 22–26.
- “Questions & Answers for applicants, marketing authorisation holders of medicinal products and notified bodies with respect to the implementation of the Medical Devices and In Vitro Diagnostic Medical Devices Regulations ((EU) 2017/745 and (EU) 2017/746)”, EMA, Jun 23, 2021.
- “Quality documentation for medicinal products when used with a medical device – Scientific guideline”. EMA, Jan 1, 2022.
Previous article
THE END OF THE ROAD FOR pMDIs?Next article
FUTURE-PROOFING pMDI DESIGN TO FIT A CHANGING MARKET
