To Issue 151
Citation: Gross C, Tunkel M, “Chronic Patients at the Centre of Large-Volume Drug Subcutaneous Delivery with a Sustainable On-Body Injector”. ONdrugDelivery, Issue 151 (Sep 2023), pp 62–65.
Cécile Gross and Mark Tunkel discuss the role of patient-centricity in the development of Nemera’s sustainable on-body injector platform.
“A new drug delivery system for a bolus injection was needed for patients outside diabetes.”
“Putting the patient at the centre of healthcare” and “patient-centred care” are objectives that have been stated for more than two decades in the medical world, whereas “patient-centricity” has appeared in the life sciences field more recently but covers the same principles. These terms are still relevant today, as there is definitively room for improvement. This is reflected in the 2022 article from W Baldwin, Associate Director Life Sciences and Patient Experience Center of Excellence Lead at Accenture (Dublin, Ireland), evocatively titled “Why does patient centricity matter to pharma?”
It is true that, beyond the nice concepts and the humanistic vision that build community consensus, turning these principles into tangible assets is not as easy as it seems. “Zeal without knowledge is like fire without light,” as one might say. Specifically, the parenteral field is facing several challenges to achieve this goal, especially where administering large volumes at home is concerned.
Self-administration is not new in subcutaneous delivery, and neither is targeting a patient population with chronic diseases. The latest innovations in drug formulation, as well as the rise in use of biologics, have led to the development of large-volume injectors. So far, safety systems, pen injectors and autoinjectors have been constrained by the drug dose and their primary drug container fill volumes.
In parallel, large-volume injections have been performed subcutaneously for a long time in the diabetes space, coupled with continuous blood glucose management. However, the dose is variable, and the entire injection is spread over a long period of time. Thus, a new drug delivery system for a bolus injection was needed for patients outside diabetes.
To revisit the challenges mentioned above, the question is how to take a patient-centric path while considering both a novel drug delivery device and a new patient population? Nemera chose to address this question with a twin-track strategy. On the one hand, identifying the patient journey and preferences and, on the other, building a sustainable on-body injector platform (Figure 1).

Figure 1: Nemera’s Symbioze™ on-body injector.
“Skin modelling is an ongoing task at Nemera, trying to answer questions related to drug distribution, skin back pressure and pain.”
PATIENT JOURNEY AND PATIENT PREFERENCES
Four main therapeutic areas have been identified, all of which are considered to be “chronic diseases”, but with different treatment regimens, durations and injection frequencies. Nemera commissioned its Insight by Nemera capability to conduct several studies. One usability study focused on this typology of therapies. Patients were recruited in the following categories: oncology (e.g. multiple myeloma, acute myeloid leukaemia, lung cancer, breast cancer), autoimmune diseases (e.g. rheumatoid arthritis, Crohn’s disease, Ankylosing Spondylitis, atopic dermatitis, psoriasis), haematological immune deficiencies (e.g. haemophilia A) and disorders of the central nervous system (e.g. multiple sclerosis, Parkinson’s disease, Alzheimer’s disease).
Many of these patients have made the switch from intravenous to subcutaneous injection, with relief according to the latest systematic review,1 which is in line with the assessment obtained for the forerunners trastuzumab2 and rituximab.3 The primary beneficial outcomes of such a switch are time savings, which have a positive impact on daily life, and flexibility in terms of scheduling and care.4 Therefore, there is a need to understand the impact on biological tissue (Figure 2). Skin modelling is an ongoing task at Nemera, trying to answer questions related to drug distribution, skin back pressure and pain. In parallel, Nemera is also collaborating with the Subcutaneous Drug Development & Delivery Consortium (OR, US) via a peer-to-peer sharing of experience, especially in the high-dose high-volume sub-team5 for which pain is the focus of 2023.
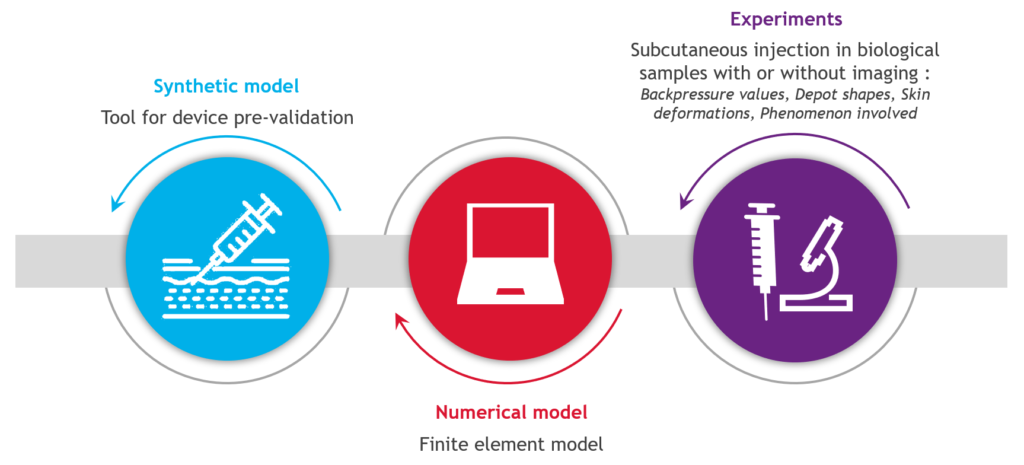
Figure 2: A skin modelling tool to understand the biological tissue impact in large-volume delivery.
“Nemera is expanding its view of the patient journey beyond the treatment journey to embrace the patient’s perspective more holistically; adding sustainability is a means for Nemera to show its commitment as a company to this topic.”
SYMBIOZE™, THE SUSTAINABLE ON-BODY INJECTOR PLATFORM
On the device side, Nemera has leveraged its experience in designing and manufacturing parenteral devices and applied it to this patient environment. The result is a two-step device following a “click, patch and go” operation. As shown in Figure 3, the reusable module must be assembled with the disposable one to perform the injection. Everything is automated once the patient presses the green button, and the disposable module comes preloaded and preset. Connectivity features are also embedded to secure the proper and complete bolus is delivered, and to allow disease management with an app keeping the link between patient and healthcare professional active.
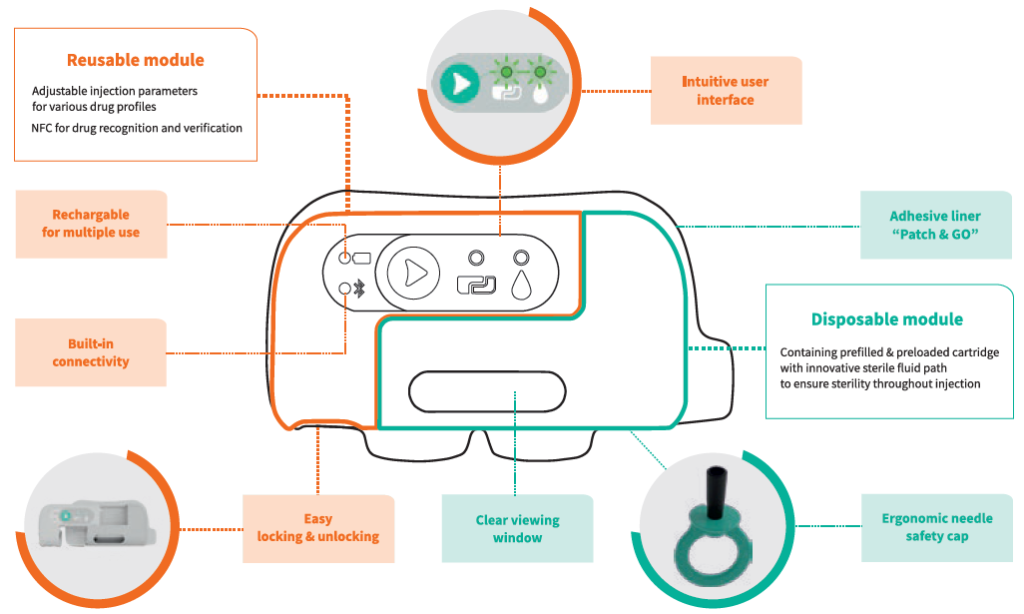
Figure 3: Symbioze™ comprises a reusable and a disposable module, offering key benefits to administer complex drugs.
“Nemera ensures that the Symbioze™ device platform is tailored to the specific needs of the patient populations, drug characteristics, delivery time and regulatory requirements of each individual project.”
To fine-tune the development of the electronics and software parts of the platform, Nemera has entered into a partnership with Zollner Elektronik AG (Zandt, Germany), one of the largest electronic manufacturing service providers. Zollner specialises in advanced mechatronics for healthcare and life sciences, railway technology, aerospace, defence, automotive technology and many other sectors. As a partner-of-choice, it will support the design, development and manufacturing of electronic drug delivery systems for both Nemera’s proprietary and customer-owned products. This collaboration will begin with Symbioze™ platform.
To quote Erwin Stöckinger, Senior Vice-President Business Division Electronics at Zollner, “From the very beginning, Nemera has been a remarkable match for Zollner on both a technological and human level for mutually developing groundbreaking solutions toward a sustainable society. [This] partnership [will] open up new growth markets.”
In the same way, Nemera is expanding its view of the patient journey beyond the treatment journey to embrace the patient’s perspective more holistically; adding sustainability is a means for Nemera to show its commitment as a company to the climate, as well as being consistent with its motto, “We put patients first”.
THE VALUE OF PARTNERING WITH AN INTEGRATED SERVICE PROVIDER
Partnering with Nemera can simplify the process of integrating the Symbioze™ platform with drug products, accelerating the development of combination products and expediting time to market while managing the complexities associated with on-body injectors. Nemera ensures that the Symbioze™ device platform is tailored to the specific needs of the patient populations, drug characteristics, delivery time and regulatory requirements of each individual project. The company’s comprehensive services and capabilities cover critical areas essential for the success of combination products:
- Analytical services and design verification
- Human factors and user experience management
- Regulatory strategy and submission authoring
- Drug/device assembly and packaging support.
Collaborating with an integrated partner provides several advantages, including a patient-centric approach that considers the device and its surrounding support elements, such as instructions for use, packaging, digital experiences (including mobile applications), and training solutions to increase adherence and engagement. Nemera recognises that navigating the complex ecosystem for combination products to achieve market success can be challenging. Its services are designed to align with the expectations of various stakeholders, including healthcare professionals, networks, payers and regulators.
To address the challenges of product development, Nemera leverages its end-to-end expertise throughout the process, ensuring consistent execution. Its approach minimises the risk of essential information being lost at different stages or when working with multiple partners. With Nemera’s experience in managing complex user needs and regulatory requirements, the company can accelerate its customers’ time to filings and market entry.
Ultimately, the value of Nemera’s integrated services lies in the flexibility it offers, allowing customers to focus on their core business of drug discovery and development. As a partner and extension of customer teams, Nemera delivers best-in-class solutions, ensuring no compromises are made when collaborating with it.
Symbioze™ is a registered trademark of Nemera La Verpillière SAS in the EU.
Find out more about Symbioze™ at: www.nemera.net/products/parenteral/wearables-symbioze.
REFERENCES
- Overton PM et al, “Patient Preferences for Subcutaneous versus Intravenous Administration of Treatment for Chronic Immune System Disorders: A Systematic Review”. Patient Prefer Adherence, 2021, Vol 15, pp 811–834.
- Waller CF, Möbius J, Fuentes-Alburo A, “Intravenous and subcutaneous formulations of trastuzumab, and trastuzumab biosimilars: implications for clinical practice”. Br J Cancer, 2021, Vol 124, pp 1346–1352.
- M. Rummel et al, “Preference for subcutaneous or intravenous administration of rituximab among patients with untreated CD20þ diffuse large B-cell lymphoma or follicular lymphoma: results from a prospective, randomized, open-label, crossover study (PrefMab)” Ann Oncol, 2017, Vol 28(4), pp 836–842.
- Jackisch C et al, “White Paper on the Value of Time Savings for Patients and Healthcare Providers of Breast Cancer Therapy: The Fixed-Dose Combination of Pertuzumab and Trastuzumab for Subcutaneous Injection as an Example”, Adv Ther, 2022, Vol 39(2), pp 833–844.
- Gandhi R, Badkar A, LaBarre M, “Subcutaneous Delivery of High-Dose/Volume Biologics: Current Status and Prospect for Future Advancements”. US National Library of Medicine, National Institutes of Health, Jan 2021.

