To Issue 157
Citation: Kraus R, “Comparative Usability Study of Multidose Preservative-Free Eye Drop Devices”. ONdrugDelivery, Issue 157 (Mar 2024), pp 18–22.
Rouven Kraus discusses the results of a study investigating dose accuracy and reproducibility, along with user preferences, for a variety of multidose preservative-free eye drop devices.
“Non-adherence to prescribed medication regimens can occur unintentionally when medication is not administered correctly.”
INTRODUCTION
The administration of ophthalmic drugs presents a multifactorial challenge for numerous patients suffering from dry eye disease, glaucoma or any other ophthalmic-associated disease that requires the use of topical eye drops. Non-adherence to prescribed medication regimens can occur unintentionally when medication is not administered correctly.1 In the dry eye field, with medications such as sodium hyaluronate to relieve eye dryness and soreness, unintentional non-adherence can be avoided by simply dispensing an additional eye drop, even if this results in a more costly approach for the patient. In glaucoma therapies, however, non-adherence poses a more significant concern. These kinds of medications are classified as a drug product with stricter regulations in regard to compliance.
STUDY OBJECTIVE
The independent medical usability agency, Custom Medical (Darmstadt, Germany), performed a comparative usability study with several commercialised eye drop devices using multidose preservative-free technologies (MDPF). Custom Medical is a well-known agency certified by ISO 13485, offering EU Medical Device Regulation (MDR)- and US FDA-compliant usability and human factors engineering studies and user interface design. For this study, the emphasis was placed on evaluating dose accuracy and reproducibility among the tested devices. The study involved a multinational benchmark assessment with lay persons possessing either US or European citizenships.
METHODOLOGY
The study included four MDPF eye drop devices from different manufacturers – the 3K®-Pump Eye Dropper with Standard and ComfortGrip finger sleeves from Aero Pump, the Ophthalmic Squeeze Dispenser (OSD) from Aptar Pharma, and Novelia® from Nemera. The participants, who were evenly distributed between the US and Europe, were predominantly female and represented diverse age groups, and engaged in two tasks per test device. The tasks simulated scenarios of first use and repeated use, focusing on the precise dispensing of one drop onto protective goggles and targeted surfaces.
TEST DEVICES
Testing included a total of eight different types of MDPF eye drop devices, of which five were based on different designs of Aero Pump’s 3K®-Pump Eye Dropper. The focus of this study lay on the four MDPF devices described in Table 1.
| # | Device name | Accessory | Manufacturer |
| 1 | 3K®-Pump Eye Dropper | Standard (cylindric) finger sleeve |
Aero Pump |
| 2 | 3K®-Pump Eye Dropper | ComfortGrip finger sleeve | Aero Pump |
| 3 | OSD | – | Aptar Pharma |
| 4 | Novelia® | – | Nemera |
Table 1: Test devices.
PARTICIPANT DEMOGRAPHICS
US Citizens
- 50% male/50% female
- 10% aged 20–45 years
- 30% aged 46–60 years
- 60% aged 61+ years
EU Citizens
- 30% male/70% female
- 20% aged 20–45 years
- 30% aged 46–60 years
- 50% aged 61+ years
According to demographic statistics, the majority of ophthalmic disorders are more prevalent in older individuals and tend to affect women more frequently than men.2 These gender and age factors were considered when selecting the subject group for this comparison study.
The entire study was monitored by qualified persons, who posed specific tasks and questions to the lay person participants. Eight questions were posed in total, two of which have not been included in the final statistics. The focus of the six included questions was to gather data on the participant’s preferences for the different MDPF eye drop devices tested.
“The majority of ophthalmic disorders are more prevalent in older individuals and tend to affect women more frequently than men.”
RESULTS
The study evaluated the participants’ experiences and preferences using a 10-step scale across six questions addressing various aspects of device usability. Overall, the 3K®-Pump Eye Dropper with ComfortGrip finger sleeve received the highest ratings.
Simulation of First Use and Repeated Use Scenarios
The target of the test was for participants to precisely dispense one drop from the test devices in two differing scenarios. For the first task, participants were tasked to drip exactly one drop onto the right side of a pair of worn safety goggles and one drop on the left side of the goggles (Figure 1). For compliance reasons, the participants did not have to dispense the drops into their eyes, but onto the worn protective goggles.

Figure 1: Dispensing onto eye/protective goggles.
For the second task, participants were asked to drip exactly one drop into each of the eight circles on a target surface (Figure 2). The target surfaces were based on 2 cm-sized round circles to represent the size of a human eye. This test was designed to determine the dose accuracy of the devices.

Figure 2: Dispensing onto targeted surfaces.
Tasks and Questions
The following questions were rated on a 10-step scale:
Q1: “How easy or difficult was it for you to apply the drops exactly into your eye or safety goggles?”
Question one was focused on the dose accuracy of the dispensing system. The US participants found Novelia® to be most exact, while the EU participants preferred the 3K®-Pump with ComfortGrip finger sleeve (Figure 3).
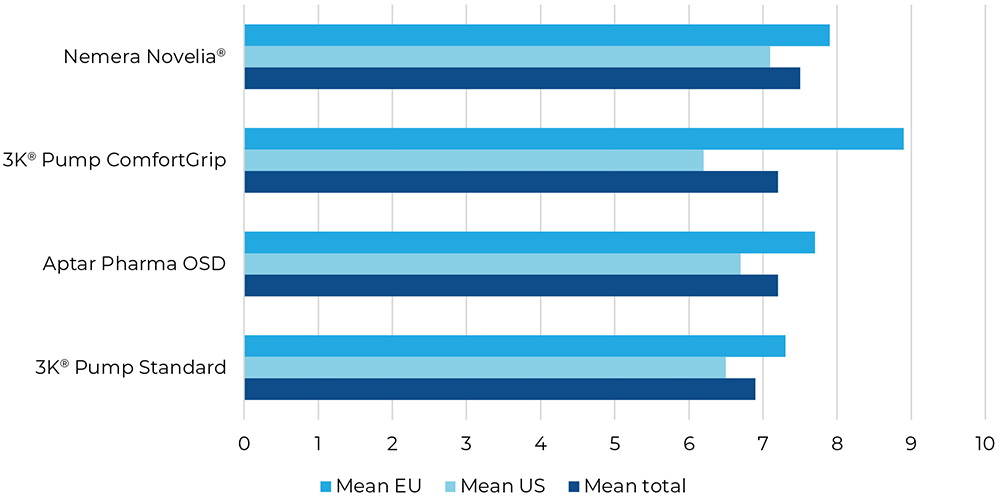
Figure 3: Result for “How easy or difficult was it for you to apply the drops exactly into your eye or safety goggles?”
Q2: “How easy or difficult was it for you to dispense precisely one drop for all applications?”
Question two was focused on the dose control, ensuring that just one single drop was dispensed per activation. The 3K®-Pumps were ranked by far the best (Figure 4).
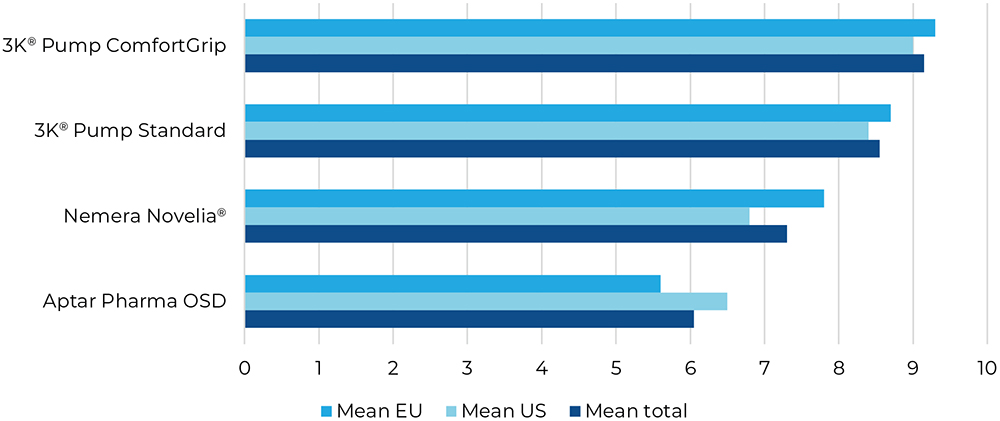
Figure 4: Result for “How easy or difficult was it for you to dispense precisely one drop for all applications?”
Q3: “How consistent did you find the size of the drops?”
Question three assessed dose consistency, which is strictly regulated by pharmacopeial guidelines. In this study, the 3K®-Pumps were perceived to create the most consistent drops overall (Figure 5).
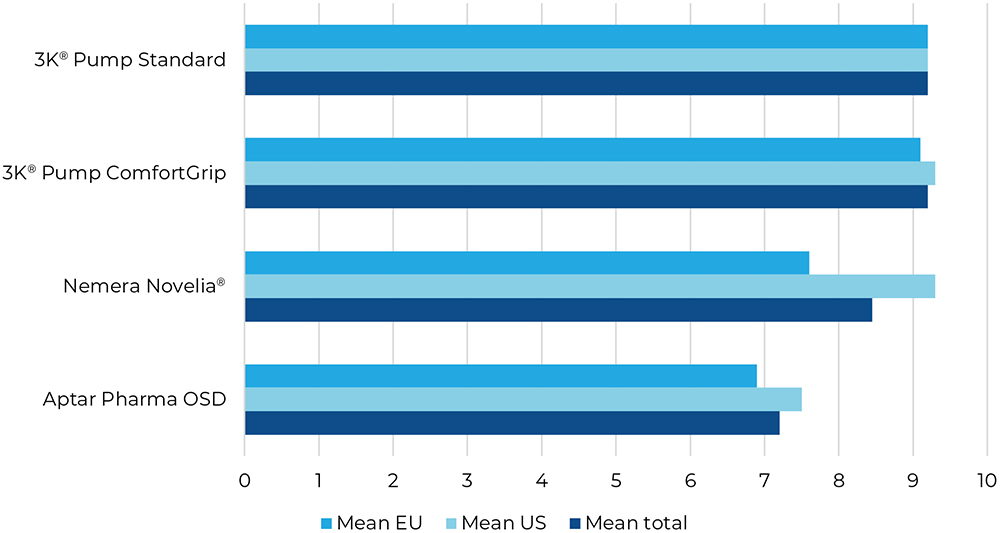
Figure 5: Result for “How consistent did you find the size of the drops?”
Q4: “How high do you consider the risk of incorrect use of this dropper to be?”
Question four was focused on safety – risk assessment is also a key factor for patients to choose the right product for them. The 3K®-Pumps were perceived to be the most safe (Figure 6).
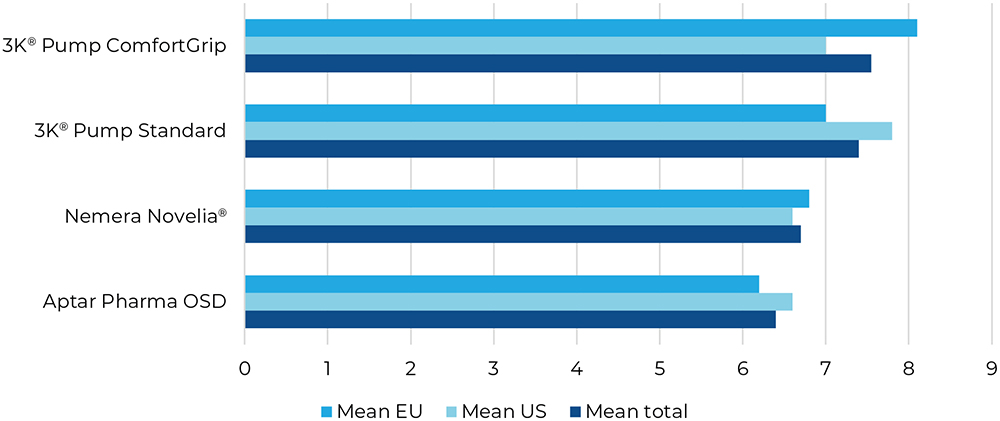
Figure 6: Result for “How high do you consider the risk of incorrect use of this dropper to be?”
Q5: “How well does the dropper fit in your hand?”
Question five considered usability based on the test devices’ sizes and forms. In this study, the average participant said that the squeeze-type devices fitted better in their hands. However, there was a clear preference of the EU participants towards the 3K®-Pump with the ComfortGrip finger sleeve (Figure 7).
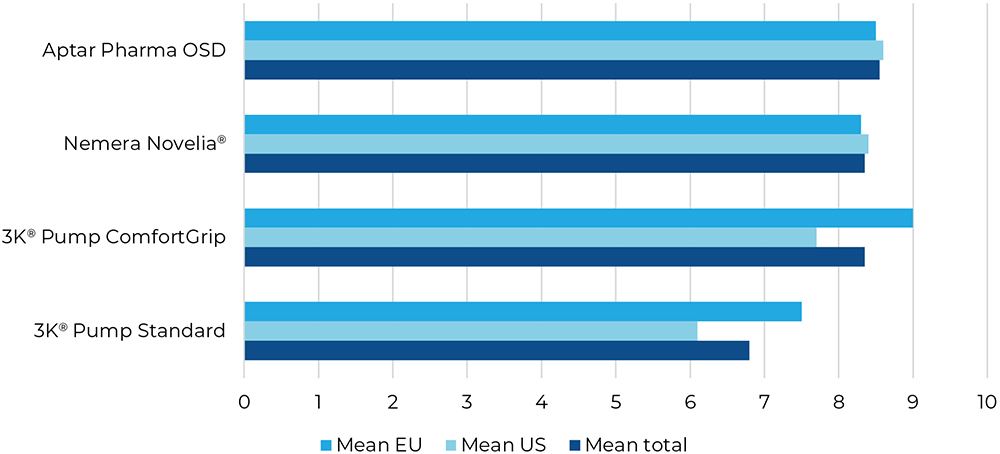
Figure 7: Result for “How well does the dropper fit in your hand?”
Q6: “How do you like the product’s appearance?”
Question six focused on the test devices’ aesthetics. The OSD’s appearance was preferred by all participant groups, followed by the 3K®-Pump with ComfortGrip sleeve (Figure 8).
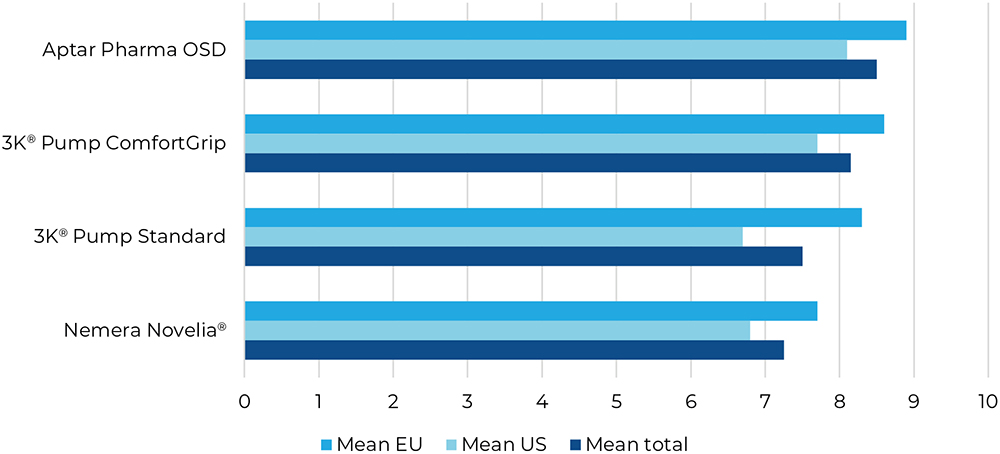
Figure 8: Result for “How do you like the product’s appearance?”
“Widely used for both over-the-counter and prescription medications, the 3K®-Pump is an established and trusted choice for
ophthalmic medications.”
Taking all these results into consideration by compiling the ratings of all six questions (Table 2), the 3K®-Pump with ComfortGrip finger sleeve received the best overall rating, followed by the 3K®-Pump with Standard finger sleeve (Figure 9).
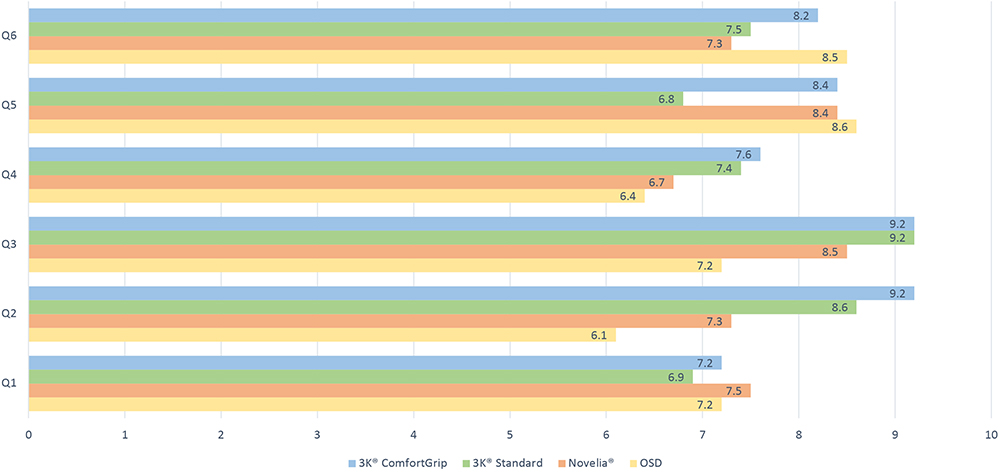
Figure 9: Overall rating.
| 3K® ComfortGrip | 3K® Standard | Novelia® | OSD | |
| Q1 | 7.2 | 6.9 | 7.5 | 7.2 |
| Q2 | 9.2 | 8.6 | 7.3 | 6.1 |
| Q3 | 9.2 | 9.2 | 8.5 | 7.2 |
| Q4 | 7.6 | 7.4 | 6.7 | |
| Q5 | 8.4 | 6.8 | 8.4 | 8.6 |
| Q6 | 8.2 | 7.5 | 7.3 | 8.5 |
| Mean | 8.3 | 7.7 | 7.6 | 7.3 |
Table 2: Overall rating.
The final question posed to the participants asked them to come to a conclusion on which of the test devices they thought would minimise the risk of overdosing. The majority of the participants preferred a metered dose pump over a squeeze dispenser (Figure 10).
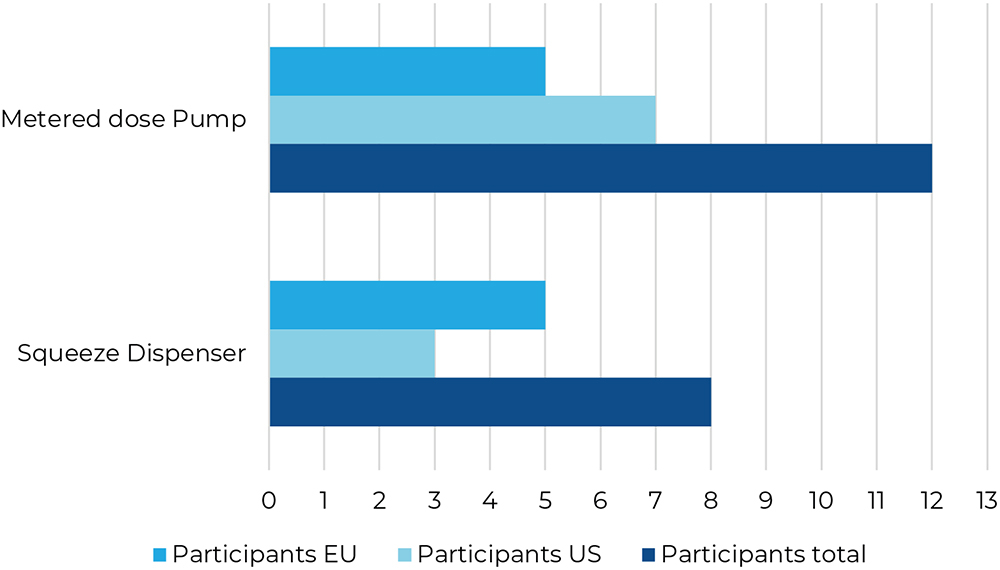
Figure 10: Risk of overdosing, select one particular device.
CONCLUSION
This study’s findings underscore the importance of taking usability factors into account when designing eye drop devices, particularly for ensuring dose accuracy and user preference. The study highlighted the potential of metered dose pump devices, such as Aero Pump’s 3K®-Pump, to enhance patient adherence and satisfaction in ophthalmic medication administration.
AERO PUMP’S PRESERVATIVE-FREE OPHTHALMIC MULTIDOSE SYSTEM
Aero Pump’s 3K®-Pump Eye Dropper has become well-established on the market since its first product launch in 2006. It is the only available eyedropper based on a purely mechanic pump technology that offers a metered dose. The 3K® System incorporates specialised components that actively avoid microbial ingress, ensuring microbiological safety. Widely used for both over-the-counter and prescription medications, the 3K®-Pump is an established and trusted choice for ophthalmic medications.
With a focus on user convenience, the 3K® System offers various user-friendly actuation aids to facilitate effortless application directly into the patient’s eye. Pharmaceutical manufacturers can choose their preferred finger sleeve design, which can be customised according to the needs of the end user (Figure 11).

Figure 11: Aero Pump’s ophthalmic multidose system in different sleeve designs.
Flexible in its compatibility, the pump system can be adapted for use with both plastic and glass containers and can accommodate various fill sizes. This versatility minimises interactions between the packaging materials and the drug substance, offering distinct advantages.
Maintaining precision throughout its lifecycle, the 3K® System dispenses an accurate dose with each stroke, providing consistent performance. The actuation force of Aero Pump’s ophthalmic multidose system remains stable regardless of the residual liquid inside the container.
For drug-device combination products, Aero Pump can provide a CE-marked product as a medical device compliant with the EU MDR. This facilitates and expedites the approval process for the finished drug product, and further avoids additional costs for a notified body opinion. Aero Pump offers comprehensive support for a successful regulatory submission process in markets worldwide.
REFERENCES
- Baker-Schena L, “Patient Adherence”. EyeNet Magazine, Sep 2017, pp 51–55.
- Dalton M, “Understanding prevalence, demographics of dry eye disease”. Ophthalmology Times, Jul 2019.

