Citation: Decock T, Gremillet H “Connected Devices – Medication Just Got Personal”. ONdrugDelivery Magazine, Issue 98 (Jun 2019), pp 58-61.
Thierry Decock and Hadrien Gremillet explore how the obstacles to the adoption of connectivity can be overcome and how connected devices can deliver truly patient-centric care that will clearly improve adherence rates, whilst demonstrating a clear cost benefit to the pharmaceutical partner.
Personalisation has become an essential component in people’s everyday lives and technology is a key enabler of the ability of drug delivery device and pharma companies to deliver that level of personalisation. From wellbeing apps to connected homes, people are demanding technology that serves them personally. It could be argued that for people with chronic conditions there is no more important area of their lives where personalisation can make a difference than their medication.
It is no secret that low patient adherence has been identified as a substantial problem, causing significant yet avoidable healthcare costs to payers and healthcare systems and, most urgently, impacting quality of life for affected patients failing to control their conditions.
Whilst the concept of connected devices is widely accepted, barriers to the implementation of such technology remain, in particular regarding the demonstration of cost versus benefit, and the transference of technology across different therapy areas.
It’s impossible to deny that poor patient adherence is a problem. Yet there is a significant issue in defining its true economic impact because differences in the methods adopted by various studies make understanding the true picture difficult. For example, one source estimates the annual costs of medication non-adherence range from US$100-290 billion (£79-229 billion) in the US1 whereas another estimates the equivalent figure for Europe to be just €1.25 billion (£1.11 billion).2 It is highly unlikely that the cost of non-adherence in Europe is really 100-fold smaller than in the US, but differing analytical methods have led to wildly different estimates. All the time there is no universally agreed measure, the argument for defined investment in a solution such as connectivity can always be challenged.
Nevertheless, even though the figures for cost vary, the abundant data on the scale of non-adherence are consistent in that they paint a picture of a significant problem. For example, worldwide, nearly half of all adults and approximately 8% of children aged 5–17 years have a chronic condition3 and it is estimated that non-adherence can be as much as 50% in long-term therapies for chronic illnesses.4 10% of hospitalisations in older adults are attributed to medication non-adherence5,6 resulting in three extra medical visits per year and an increased annual treatment cost of US$2,000.7 In diabetes, the estimated cost savings associated with improving adherence range from $661 million to $1.16 billion.8 And these data don’t count the personal cost to patients who, for any number of reasons, fail to take their medication properly.
DIGITAL MEDTECH DELIVERS ANSWERS
It is interesting to note that connected drug delivery devices themselves are likely by far the best, cheapest, most reliable means available to us for gathering the very data that would demonstrate most reliably, accurately and in the most detail, the true extent, nature and cost of non-adherence. This situation could be seen to represent a frustrating “Catch 22” – the best way of gathering the data that provides the evidence supporting the adoption of connectivity is to adopt connectivity.
At first glance it is almost impossible to quantify because the market is so immature – all of the device manufacturing companies have invested heavily in concept creation, but there are still relatively few connected drug delivery devices on the market. That said, as connected technology is gradually being adopted in the drug delivery sector, the body of knowledge about non-adherence is beginning to grow accordingly. And we can learn from other innovations, such as visual reminders, and the impact they have had on adherence and cost benefit to the healthcare community.
For example, data from electronic monitoring systems has revealed that there are six patterns of patient adherence-resulting in “the rule of one sixth”. Approximately one sixth of patients come close to perfect adherence; one sixth take nearly all doses, but with irregular timing; one sixth miss an occasional single daily dose and have some timing irregularity; one sixth take drug breaks every three to four months, with occasional omissions of doses; one sixth have one or more drug breaks monthly, with frequent omissions of doses; and one sixth take few or no doses, but may report that they are compliant.9
A literature review, which examined the effect of reminder systems on patient adherence to treatment, 11 published randomised controlled trials were found between 1999 and 2009 which measured adherence to a daily medication. Analysis showed a statistically significant increase in adherence in groups receiving a reminder intervention compared with controls (66.61% versus 54.71%). Eight of the 11 studies showed a statistically significant increase in adherence for at least one of the reminder group arms compared with the control groups receiving no reminder intervention.10
Chronic management of asthma is typically associated with adherence rates of less than 50%, with increased risk of mortality, meaning that both the scope for improving adherence in this area, and the benefits of doing so, are clear. Charles et al investigated whether an audio-visual reminder could improve adherence to inhaled corticosteroid therapy in asthma. In the study, 110 adults and adolescents with asthma were randomised to receive 24 weeks of inhaler therapy via a metered-dose inhaler with or without an audio-visual reminder function.
The study demonstrated a positive benefit of the reminder system. The absolute difference in median percentage adherence between the two groups was 18% (95% CI, 10-26%; P <.0001). Furthermore, the proportion of subjects taking >50% of their medication was 95.5% in the intervention group compared with 71.7% in the control group. The proportion of subjects taking >80% of their medication was 88.6% in the intervention group, compared with 39.1% in the control group. Finally, the proportion of subjects taking more than 90% of their medication was 63.6% in the intervention group compared with 19.6% in the control group, an approximately three-fold improvement.11
INDUSTRY ADOPTION IS UNDERWAY
In 2018, The Deloitte Center for Health Solutions and the US Advanced Medical Technologies Association (AdvaMed) surveyed 22 medtech companies to understand how R&D leaders are responding to key trends such as the need to improve efficiency and reduce costs as health systems, patients, and payers require evidence to justify product value and reimbursement. All companies surveyed reported investing in device connectivity, with 73% expecting R&D spending on software development to be more than 10% of budgets by 2020.12
Companies are investing more than ever in connectivity as evidence detailing the economic consequences of non-adherence continue to grow. Haynes et al went as far as to suggest that “increasing the effectiveness of adherence interventions may have a far greater impact on the health of the population than any improvement in specific medical treatments.”13
Some drug delivery device companies, such as Nemera, are already ahead of the curve. Renowned for delivering patient-centric device designs, Nemera has already developed proof-of-principle connected devices for nasal and ophthalmic drug delivery as well as injection. The company has invested in connected and smart devices based on the strong belief that the technology directly benefits patients by improving adherence.
Value Through Application Across Different Therapy Areas
Referring back to the Deloitte study, it is anticipated that, by 2020, companies will be devoting a significant portion of R&D budgets (>10%) to software development.12 Nemera believes that investment can be maximised by efficiently delivering common technological “building bricks” that can serve multiple device categories, themselves each serving a still larger number of therapy areas.
Nemera has an established global reputation in, and a first-class device portfolio spanning, the parenteral, nasal, pulmonary and ophthalmic delivery device categories, amongst others. The company manufactures hundreds of millions of devices and components each year, for numerous therapeutic markets worldwide.
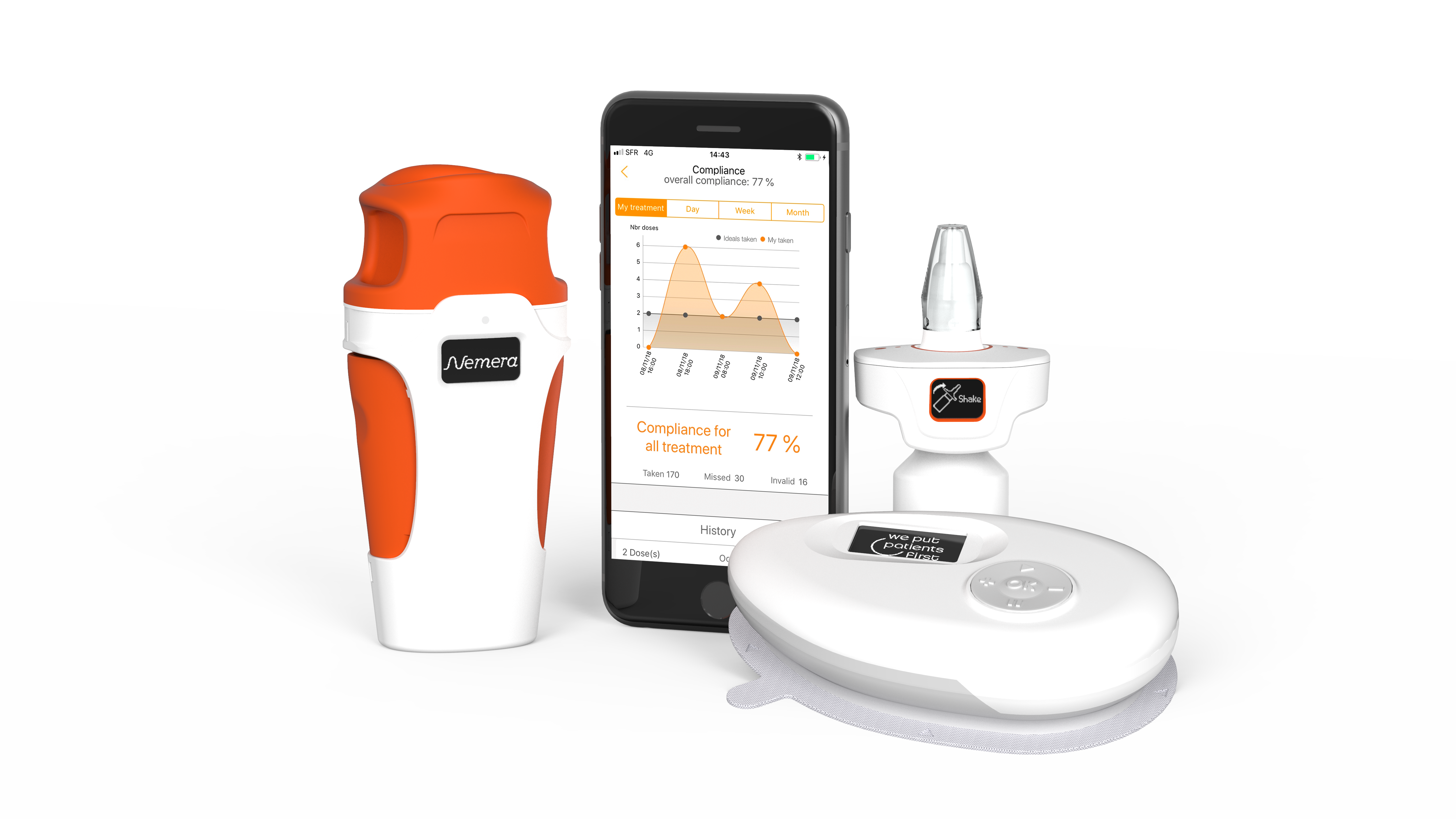
Figure 1: Electronic features are transferable across multiple device platforms such as ophthalmic, nasal and parenteral.
In the same way that Nemera uses its expertise and know-how built up in one drug delivery device category to inform developments in others, the technical and functional knowledge developed around connectivity as applied to one device can be leveraged for others. The e-Novelia® connected add-on for ophthalmic delivery, for example, delivers adherence monitoring, dosage history, digital tutorials and medication reminders – designed to improve the personal experience and increase adherence. This knowledge and experience has been, and is being, applied in the development of connected nasal (e-Advancia®) and injection devices, amongst others. The ability to transfer the technology wherever it’s needed to additional device categories and additional therapy areas increases efficiency, drives down costs, and maximises the return on R&D investment (Figure 1).
Users Contribute to Device Aesthetics and Functionality
The best way to deliver connected devices that can truly personalise the treatment experience is to ask the people who are going to use it what they want. This is a key characteristic of Nemera’s approach – consulting both clinicians and patients in the early phases of development. There is a clear consensus that traditional considerations such as ergonomics and handling continue to be important, but putting smart novel technology in front of users provides new insights and reveals different wants and needs. Examples include the desire to replace paper prescriptions with automated electronic ones, and ideas such as step-by-step video instructions on use, rather than complicated guidance notes, are also frequently mentioned. Overall the message from users is clear – enable us to take control of our own care.
New Payment Models Influence a Wave of Innovation
In addition to personalising the device user experience, connectivity is already beginning to influence industry commercial practices and processes. For example, connectivity facilitates the adoption of novel payment / reimbursement models, enabling them to be personalised in that they can be tailored to individual patient behaviours (including adherence and compliance) and even treatment outcomes.
The Deloitte study revealed some interesting perspectives. 68% of surveyed companies are shifting their focus on innovation because of reforms to payment models and care delivery. These payment models can positively impact both caregivers and patients. For hospitals and clinicians, incentives to deliver better quality care while also delivering better value are being explored with metrics such as improved outcomes, reduced costs, decreased post-treatment complications and increased procedural efficiency cited as motivations for this move. 64% of surveyed companies also reported discussing, piloting or implementing value-based contracts with payers or providers, all tied closely to device performance and patient outcomes where patients are rewarded for greater adherence.
CONCLUSION
Whilst barriers to their adoption exist, not least around the justification of the cost versus benefit, connected drug delivery devices undoubtedly present opportunities to tackle the vast problem of medication non-adherence, to facilitate novel payment models, and to take the personalisation of medication to a new level not previously imaginable in the pre-connectivity era.
As an early adopter, with the ability to leverage know-how and capabilities across a broad technology portfolio spanning most drug delivery categories, and transfer technological advances across numerous therapy areas, Nemera is perfectly positioned to overcome the barriers that remain, maximising the return on investment in R&D, driving the development of connected drug delivery devices, and their subsequent adoption by industry. The potential for improving patient quality of life and health outcomes, and equally the impact on healthcare costs, is compelling.
REFERENCES
- “Adherence to long term therapies; evidence for action”. Research Report, World Health Organization, January 2003.
- “Targeting adherence. Improving patient outcomes in Europe through community pharmacists’ intervention”. Position Paper, Pharmaceutical Group of the European Union (PGEU), 2008
- “Thinking outside the pillbox: a system-wide approach to improving patient medication adherence for chronic disease”. Research Brief, New England Healthcare Institute, August 2009.
- US Centres for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion website. (https://www.cdc.gov/chronicdisease/ index.htm. Accessed May 2019)
- Sokol M, McGuigan K, Verbrugge R, Epstein R, “Impact of medication adherence on hospitalization risk and healthcare cost”. Med Care, 2005, Vol 43(6), pp521-530.
- Vermiere E et al, “Context and health outcomes”. Lancet, 2001, Vol 357, pp 2059-2060.
- “Medication compliance-adherence-persistence (CAP) Digest”. American Pharmacists Association (APhA) and Pfizer, 2003.
- Egede LE et al, “Medication nonadherence in diabetes: longitudinal effects on costs and potential cost savings from improvement”. Diabetes Care, 2012, Vol 35, pp 2533-2539.
- Figge H, “Electronic Tools to Measure and Enhance Medication Adherence”. US Pharmacist, April 2011.
- Fenerty S et al, “The effect of reminder systems on patients’ adherence to treatment”. Patient Prefer Adherence, 2012, Vol 6, pp 127-135.
- Charles T et al, “An audiovisual reminder function improves adherence with inhaled corticosteroid therapy in asthma”. J Allergy Clin Immunol, 2007, Vol 119, pp 811-816.
- Murray B, Hakim L, Shah S, “The next wave of innovation”. Research Report, The Deloitte Center for Health Solutions and AdvaMed, 2018.
- Haynes R, McDonald H, Garg A, Montague P, “Interventions for helping patients to follow prescriptions for medications”. Cochrane Database Syst Rev, 2002, Vol 2, CD000011.

